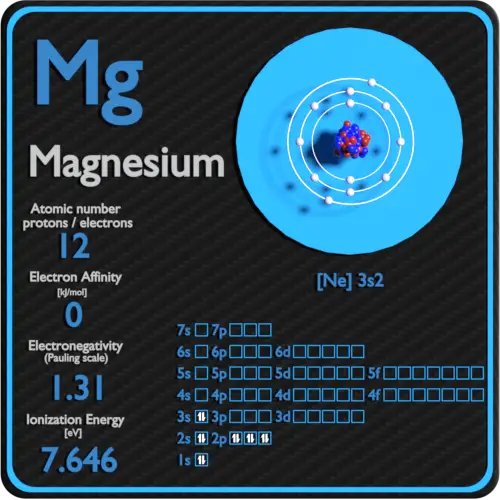
Magnesium Ion Diagram . Draw the electron configuration diagram for each atom. The valence electron configuration for magnesium is [ne] 3s², indicating that the two valence electrons are in the 3s orbital. Magnesium oxide dot and cross diagram. Understand how magnesium ions are formed. Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.check me out:. Bohr diagrams indicate how many electrons fill each principal shell. Draw the outer shell of each atom. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. [13] it is found in. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Swap the crosses for dots in one of your diagrams. Magnesium has two electrons in its outer shell, oxygen has six. Learn about the structure and properties of magnesium ions with a detailed diagram. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell.
from material-properties.org
Magnesium oxide dot and cross diagram. Understand how magnesium ions are formed. [13] it is found in. Learn about the structure and properties of magnesium ions with a detailed diagram. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. The valence electron configuration for magnesium is [ne] 3s², indicating that the two valence electrons are in the 3s orbital. Draw the electron configuration diagram for each atom. Magnesium has two electrons in its outer shell, oxygen has six. Bohr diagrams indicate how many electrons fill each principal shell. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell.
Magnesium Periodic Table and Atomic Properties
Magnesium Ion Diagram During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Draw the outer shell of each atom. Bohr diagrams indicate how many electrons fill each principal shell. Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.check me out:. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Understand how magnesium ions are formed. [13] it is found in. Magnesium oxide dot and cross diagram. Magnesium has two electrons in its outer shell, oxygen has six. Draw the electron configuration diagram for each atom. Swap the crosses for dots in one of your diagrams. The valence electron configuration for magnesium is [ne] 3s², indicating that the two valence electrons are in the 3s orbital. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. Learn about the structure and properties of magnesium ions with a detailed diagram. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium Ion Diagram Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.check me out:. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Draw the electron configuration diagram for each atom. Understand how magnesium ions are formed. Magnesium is a group 2 metal so will lose two outer. Magnesium Ion Diagram.
From mavink.com
Magnesium Atomic Structure Magnesium Ion Diagram Magnesium has two electrons in its outer shell, oxygen has six. Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.check me out:. Draw the electron configuration diagram for each atom. Draw the outer shell of each atom. Understand how magnesium ions are formed. Bohr diagrams indicate how many electrons fill each principal. Magnesium Ion Diagram.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Magnesium Ion Diagram Draw the outer shell of each atom. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Swap the crosses for dots in one of your diagrams. Magnesium oxide dot and cross diagram. Magnesium has two electrons in its outer shell, oxygen has six. Magnesium has 2 electrons in its first shell, 8 in. Magnesium Ion Diagram.
From www.pinterest.se
GensonScience Magnesium Atom model project, Atom model, Atoms and Magnesium Ion Diagram Understand how magnesium ions are formed. Draw the electron configuration diagram for each atom. [13] it is found in. Bohr diagrams indicate how many electrons fill each principal shell. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Group 18 elements (helium, neon, and argon are shown) have a full outer,. Magnesium Ion Diagram.
From www.mikrora.com
File Electron Configuration Magnesium Svg Best Diagram Collection Magnesium Ion Diagram Draw the outer shell of each atom. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Magnesium oxide dot and cross diagram. Bohr diagrams indicate how many electrons fill each principal shell. Magnesium has two electrons in its outer shell, oxygen has six. Understand how magnesium ions are formed. [13] it is found. Magnesium Ion Diagram.
From mungfali.com
Magnesium Orbital Diagram Magnesium Ion Diagram Understand how magnesium ions are formed. Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.check me out:. Draw the electron configuration diagram for each atom. [13] it is found in. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Learn about the structure and properties. Magnesium Ion Diagram.
From brainly.in
Write lewis dot structure of Mg2+ Brainly.in Magnesium Ion Diagram Understand how magnesium ions are formed. Bohr diagrams indicate how many electrons fill each principal shell. Draw the outer shell of each atom. Learn about the structure and properties of magnesium ions with a detailed diagram. [13] it is found in. Magnesium oxide dot and cross diagram. During ionic bonding the atoms form ions by gaining or losing electrons to. Magnesium Ion Diagram.
From ar.inspiredpencil.com
Magnesium Molecule Magnesium Ion Diagram Understand how magnesium ions are formed. Bohr diagrams indicate how many electrons fill each principal shell. Magnesium oxide dot and cross diagram. Learn about the structure and properties of magnesium ions with a detailed diagram. Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.check me out:. Draw the electron configuration diagram for. Magnesium Ion Diagram.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Magnesium Ion Diagram Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.check me out:. Magnesium oxide dot and cross diagram. The valence electron configuration for magnesium is [ne] 3s², indicating that the two valence electrons are in the 3s orbital. Learn about the structure and properties of magnesium ions with a detailed diagram. Magnesium is. Magnesium Ion Diagram.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Ion Diagram Understand how magnesium ions are formed. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Learn about the structure and properties of magnesium ions with a detailed diagram. Swap the crosses for dots in one of your diagrams. Magnesium oxide dot and cross diagram. Draw the outer shell of each atom.. Magnesium Ion Diagram.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Ion Diagram Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.check me out:. Bohr diagrams indicate how many electrons fill each principal shell. During ionic bonding the atoms form ions by gaining or losing electrons. Magnesium Ion Diagram.
From galvinconanstuart.blogspot.com
Electron Dot Diagram For Magnesium General Wiring Diagram Magnesium Ion Diagram Draw the outer shell of each atom. [13] it is found in. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Understand how magnesium ions are formed. Learn about the structure and properties of magnesium ions with a detailed diagram. Swap the crosses for dots in one of your diagrams. Magnesium. Magnesium Ion Diagram.
From www.flexiprep.com
NCERT Class 9 Science Solutions Chapter 3 Atoms and Molecules Part 9 Magnesium Ion Diagram [13] it is found in. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. Magnesium has two electrons in its outer shell, oxygen has six. Swap the crosses for dots in one of your diagrams. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence,. Magnesium Ion Diagram.
From nursehub.com
Electron Shells NurseHub Magnesium Ion Diagram Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. Magnesium oxide dot and cross diagram. Draw the electron configuration diagram for each atom. Learn about the structure and properties of magnesium ions with a detailed diagram. The valence electron configuration for magnesium is [ne] 3s², indicating that the two valence. Magnesium Ion Diagram.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Ion Diagram Learn about the structure and properties of magnesium ions with a detailed diagram. Bohr diagrams indicate how many electrons fill each principal shell. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Draw the outer shell of each atom. Magnesium has 2 electrons in its first shell, 8 in its second. Magnesium Ion Diagram.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Diagram Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. Draw the outer shell of each atom. [13] it is found in. Learn about the structure and properties of magnesium ions with a detailed diagram.. Magnesium Ion Diagram.
From ar.inspiredpencil.com
Magnesium Ion Lewis Dot Structure Magnesium Ion Diagram Understand how magnesium ions are formed. Bohr diagrams indicate how many electrons fill each principal shell. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. Swap the crosses for dots in one of your diagrams. Magnesium has two electrons in its outer shell, oxygen has six. Learn about the structure. Magnesium Ion Diagram.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Ion Diagram Magnesium oxide dot and cross diagram. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. Learn about the structure and properties of magnesium ions with a detailed diagram. [13] it is found in. Bohr diagrams indicate how many electrons fill each principal shell. Draw the outer shell of each atom.. Magnesium Ion Diagram.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Ion Diagram Draw the electron configuration diagram for each atom. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Magnesium oxide dot and cross diagram. Draw the outer shell of each atom. Bohr diagrams indicate how many electrons fill each principal shell. The valence electron configuration for magnesium is [ne] 3s², indicating that. Magnesium Ion Diagram.
From www.britannica.com
magnesium Description, Properties, & Compounds Britannica Magnesium Ion Diagram Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. [13] it is found in. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Understand how magnesium ions are formed. Draw the electron configuration diagram for each atom. Magnesium has 2 electrons in its. Magnesium Ion Diagram.
From galvinconanstuart.blogspot.com
Lewis Dot Diagram For Magnesium General Wiring Diagram Magnesium Ion Diagram [13] it is found in. Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.check me out:. Draw the electron configuration diagram for each atom. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. The valence electron configuration for magnesium is [ne] 3s²,. Magnesium Ion Diagram.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition and electron Magnesium Ion Diagram Magnesium oxide dot and cross diagram. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. The valence electron configuration for magnesium is [ne] 3s², indicating that the two valence electrons are in the 3s orbital. Draw the outer shell of each atom. Magnesium has 2 electrons in its first shell,. Magnesium Ion Diagram.
From www.youtube.com
Atomic Structure (Bohr Model) for Magnesium (Mg) YouTube Magnesium Ion Diagram Learn about the structure and properties of magnesium ions with a detailed diagram. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.check me out:. Magnesium has two electrons in its outer shell, oxygen has. Magnesium Ion Diagram.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Ion Diagram Magnesium oxide dot and cross diagram. Understand how magnesium ions are formed. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. [13] it is found in. Magnesium has two electrons in its. Magnesium Ion Diagram.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Magnesium Ion Diagram Draw the outer shell of each atom. Learn about the structure and properties of magnesium ions with a detailed diagram. Swap the crosses for dots in one of your diagrams. Bohr diagrams indicate how many electrons fill each principal shell. Magnesium has two electrons in its outer shell, oxygen has six. During ionic bonding the atoms form ions by gaining. Magnesium Ion Diagram.
From ar.inspiredpencil.com
Magnesium Atom Structure Magnesium Ion Diagram Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. Draw the electron configuration diagram for each atom. Draw the outer shell of each atom. Swap the crosses for dots in one of your diagrams. Learn about the structure and properties of magnesium ions with a detailed diagram. Magnesium has two. Magnesium Ion Diagram.
From galvinconanstuart.blogspot.com
Lewis Dot Diagram For Magnesium General Wiring Diagram Magnesium Ion Diagram Understand how magnesium ions are formed. Learn about the structure and properties of magnesium ions with a detailed diagram. Magnesium has two electrons in its outer shell, oxygen has six. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. Draw the outer shell of each atom. Magnesium oxide dot and. Magnesium Ion Diagram.
From stock.adobe.com
Bohr model diagram of magnesium in atomic physics Stock Vector Adobe Magnesium Ion Diagram Draw the electron configuration diagram for each atom. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Magnesium oxide dot and cross diagram. Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.check me out:. During ionic bonding the atoms form ions by gaining or losing. Magnesium Ion Diagram.
From www.pinterest.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Ion Diagram Understand how magnesium ions are formed. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. Bohr diagrams indicate how many electrons fill each principal shell. The valence electron configuration for magnesium is [ne] 3s², indicating that the two valence electrons are in the 3s orbital. Swap the crosses for dots. Magnesium Ion Diagram.
From www.thesciencehive.co.uk
The Periodic Table (GCSE) — the science hive Magnesium Ion Diagram During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. [13] it is found in. Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.check me out:. Magnesium has two electrons in its outer shell, oxygen has six. Draw the outer shell of each atom.. Magnesium Ion Diagram.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Ion Diagram Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Understand how magnesium ions are formed. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. Magnesium has two electrons in its outer shell, oxygen has six. Magnesium oxide dot and cross diagram. Draw the. Magnesium Ion Diagram.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Ion Diagram Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Magnesium oxide dot and cross diagram. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. The valence electron configuration for magnesium is [ne] 3s², indicating that the two valence electrons are in the 3s. Magnesium Ion Diagram.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Magnesium Ion Diagram Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.check me out:. Swap the crosses for dots in one of your diagrams. Learn about the structure and properties of magnesium ions with a detailed diagram. Magnesium oxide dot and cross diagram. Magnesium is a group 2 metal so will lose two outer electrons. Magnesium Ion Diagram.
From manualdbaldridge.z5.web.core.windows.net
Magnesium Ion Atom Diagram Magnesium Ion Diagram Magnesium has two electrons in its outer shell, oxygen has six. Learn about the structure and properties of magnesium ions with a detailed diagram. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Swap the crosses for dots in one of your diagrams. Draw the outer shell of each atom. [13]. Magnesium Ion Diagram.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Diagram During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. [13] it is found in. Draw the outer shell of each atom. Understand how magnesium ions are formed. Magnesium oxide dot and cross diagram. Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third.check me. Magnesium Ion Diagram.