Bromine Isotopes Ratio . For compounds of chlorine and. Bromine isotopes refer to the two stable isotopes of bromine, 79br and 81br, with relative mass abundances of 50.686% and 49.314%. List, data and properties of all known isotopes of bromine. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. All atomic nuclei of the chemical element bromine are summarized under. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. Isotope ratio of inorganic bromides was determined relative to isotope composition of standard mean ocean bromide (smob). Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : An absolute value is obtained for the isotopic abundance ratio of bromine using thermal emission mass spectrometers calibrated for. 49.5 if you want to be fussy!). That means that a compound containing 1 bromine atom will have two peaks in.
from periodictableguide.com
Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : All atomic nuclei of the chemical element bromine are summarized under. 49.5 if you want to be fussy!). That means that a compound containing 1 bromine atom will have two peaks in. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Isotope ratio of inorganic bromides was determined relative to isotope composition of standard mean ocean bromide (smob). Bromine isotopes refer to the two stable isotopes of bromine, 79br and 81br, with relative mass abundances of 50.686% and 49.314%. List, data and properties of all known isotopes of bromine. For compounds of chlorine and. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations.
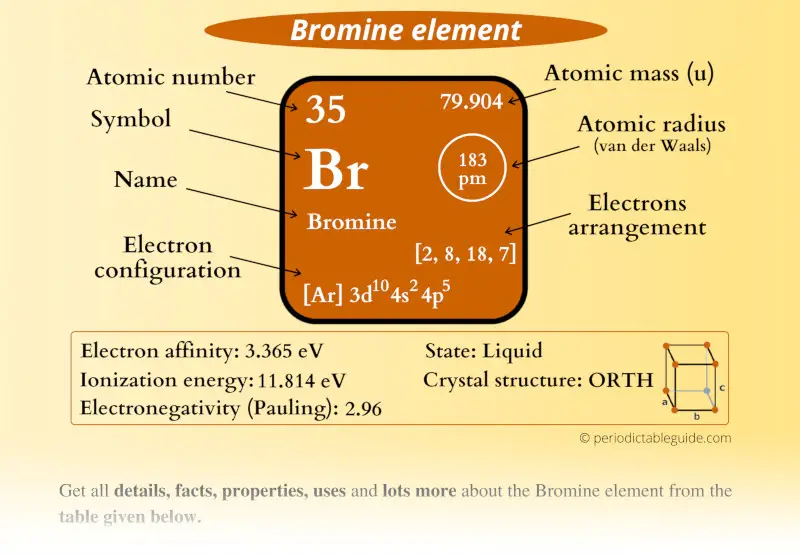
Bromine (Br) Periodic Table (Element Information & More)
Bromine Isotopes Ratio Bromine isotopes refer to the two stable isotopes of bromine, 79br and 81br, with relative mass abundances of 50.686% and 49.314%. All atomic nuclei of the chemical element bromine are summarized under. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. For compounds of chlorine and. 49.5 if you want to be fussy!). Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : Bromine isotopes refer to the two stable isotopes of bromine, 79br and 81br, with relative mass abundances of 50.686% and 49.314%. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. An absolute value is obtained for the isotopic abundance ratio of bromine using thermal emission mass spectrometers calibrated for. That means that a compound containing 1 bromine atom will have two peaks in. Isotope ratio of inorganic bromides was determined relative to isotope composition of standard mean ocean bromide (smob). List, data and properties of all known isotopes of bromine.
From www.doubtnut.com
Bromine occurs in nature mainly in the form of two isotopes Br(35)^(79 Bromine Isotopes Ratio 49.5 if you want to be fussy!). Isotope ratio of inorganic bromides was determined relative to isotope composition of standard mean ocean bromide (smob). For compounds of chlorine and. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. That means that a compound containing 1 bromine atom will. Bromine Isotopes Ratio.
From www.researchgate.net
Patterns of the molecular ion with multiple chlorine or bromine atoms Bromine Isotopes Ratio All atomic nuclei of the chemical element bromine are summarized under. For compounds of chlorine and. Isotope ratio of inorganic bromides was determined relative to isotope composition of standard mean ocean bromide (smob). That means that a compound containing 1 bromine atom will have two peaks in. List, data and properties of all known isotopes of bromine. This table shows. Bromine Isotopes Ratio.
From www.numerade.com
There are two different isotopes of bromine atoms. Under normal Bromine Isotopes Ratio This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. List, data and properties of all known isotopes of bromine. That means that a compound containing 1 bromine atom will have two peaks in. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is. Bromine Isotopes Ratio.
From www.numerade.com
SOLVED There are two different isotopes of bromine atoms. Under normal Bromine Isotopes Ratio Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : Isotope ratio of inorganic bromides was determined relative to isotope composition of standard mean ocean bromide (smob). For compounds of chlorine and. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. 49.5. Bromine Isotopes Ratio.
From www.numerade.com
Bromine has two naturally occurring isotopes (Br79 and Br81) and an Bromine Isotopes Ratio Isotope ratio of inorganic bromides was determined relative to isotope composition of standard mean ocean bromide (smob). List, data and properties of all known isotopes of bromine. That means that a compound containing 1 bromine atom will have two peaks in. 49.5 if you want to be fussy!). Bromine has two isotopes, 79 br and 81 br in an approximately. Bromine Isotopes Ratio.
From www.youtube.com
Notation for Isotopes of Bromine (Br) YouTube Bromine Isotopes Ratio 49.5 if you want to be fussy!). That means that a compound containing 1 bromine atom will have two peaks in. Bromine isotopes refer to the two stable isotopes of bromine, 79br and 81br, with relative mass abundances of 50.686% and 49.314%. All atomic nuclei of the chemical element bromine are summarized under. This table shows information about naturally occuring. Bromine Isotopes Ratio.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Bromine Isotopes Ratio This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. All atomic nuclei of the chemical element bromine are summarized under. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. An absolute value is obtained for the isotopic abundance. Bromine Isotopes Ratio.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Bromine Isotopes Ratio 49.5 if you want to be fussy!). 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. Bromine isotopes refer to the two stable isotopes of bromine, 79br and 81br, with relative mass abundances of 50.686% and 49.314%. This table shows information about naturally occuring isotopes, their atomic masses, their natural. Bromine Isotopes Ratio.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Bromine Isotopes Ratio For compounds of chlorine and. Bromine isotopes refer to the two stable isotopes of bromine, 79br and 81br, with relative mass abundances of 50.686% and 49.314%. List, data and properties of all known isotopes of bromine. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : An absolute value is obtained for the isotopic. Bromine Isotopes Ratio.
From www.istockphoto.com
Bromine Isotopes Stock Illustration Download Image Now Isotope Bromine Isotopes Ratio For compounds of chlorine and. List, data and properties of all known isotopes of bromine. 49.5 if you want to be fussy!). All atomic nuclei of the chemical element bromine are summarized under. That means that a compound containing 1 bromine atom will have two peaks in. An absolute value is obtained for the isotopic abundance ratio of bromine using. Bromine Isotopes Ratio.
From slideplayer.com
Advanced Pharmaceutical Analysis Mass spectrometry ppt download Bromine Isotopes Ratio For compounds of chlorine and. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. An absolute value is obtained for the isotopic abundance ratio of bromine using thermal emission. Bromine Isotopes Ratio.
From periodictableguide.com
Bromine (Br) Periodic Table (Element Information & More) Bromine Isotopes Ratio This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. That means that a compound containing 1 bromine atom will have two peaks in. All atomic nuclei of the chemical. Bromine Isotopes Ratio.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear Bromine Isotopes Ratio List, data and properties of all known isotopes of bromine. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : 49.5 if you want to be fussy!). An absolute value is obtained for the isotopic abundance ratio of bromine using thermal emission mass spectrometers calibrated for. All atomic nuclei of the chemical element bromine. Bromine Isotopes Ratio.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation ID2894903 Bromine Isotopes Ratio Bromine isotopes refer to the two stable isotopes of bromine, 79br and 81br, with relative mass abundances of 50.686% and 49.314%. 49.5 if you want to be fussy!). This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. All atomic nuclei of the chemical element bromine are summarized under.. Bromine Isotopes Ratio.
From www.researchgate.net
Chlorine/bromine isotope ratios (IR 37 Cl/ 35 Cl or 81 Br/ 79 Br) of Bromine Isotopes Ratio An absolute value is obtained for the isotopic abundance ratio of bromine using thermal emission mass spectrometers calibrated for. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. Isotope ratio of inorganic bromides was determined relative to isotope composition of standard mean ocean bromide (smob). For compounds of chlorine and.. Bromine Isotopes Ratio.
From vdocuments.mx
Use of the bromine isotope ratio in HPLCICPMS and HPLCESIMS Bromine Isotopes Ratio An absolute value is obtained for the isotopic abundance ratio of bromine using thermal emission mass spectrometers calibrated for. For compounds of chlorine and. Bromine isotopes refer to the two stable isotopes of bromine, 79br and 81br, with relative mass abundances of 50.686% and 49.314%. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is. Bromine Isotopes Ratio.
From www.academia.edu
(PDF) Determination of Bromine Stable Isotopes Using ContinuousFlow Bromine Isotopes Ratio An absolute value is obtained for the isotopic abundance ratio of bromine using thermal emission mass spectrometers calibrated for. That means that a compound containing 1 bromine atom will have two peaks in. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. 49.5 if you want to be fussy!). All. Bromine Isotopes Ratio.
From nap.nationalacademies.org
Table of Isotopes of Fluorine, Chlorine, Bromine and Iodine The Bromine Isotopes Ratio List, data and properties of all known isotopes of bromine. All atomic nuclei of the chemical element bromine are summarized under. That means that a compound containing 1 bromine atom will have two peaks in. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Bromine isotopes refer to. Bromine Isotopes Ratio.
From www.numerade.com
SOLVEDBromine has two naturally occurring isotopes. One of them Bromine Isotopes Ratio 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. For compounds of chlorine and. Isotope ratio of inorganic bromides was determined relative to isotope composition of standard mean ocean. Bromine Isotopes Ratio.
From giobudlfo.blob.core.windows.net
Bromine80 Isotope Notation at Eleanor Price blog Bromine Isotopes Ratio 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. That means that a compound containing 1 bromine atom will have two peaks in. 49.5 if you want to be fussy!). List, data and properties of all known isotopes of bromine. An absolute value is obtained for the isotopic abundance ratio. Bromine Isotopes Ratio.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Bromine Isotopes Ratio 49.5 if you want to be fussy!). Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : List, data and properties of all known isotopes of bromine. Bromine isotopes refer to the two stable isotopes of bromine, 79br and 81br, with relative mass abundances of 50.686% and 49.314%. For compounds of chlorine and. Isotope. Bromine Isotopes Ratio.
From www.numerade.com
SOLVED I. Bromine has two naturally occurring isotopes with the Bromine Isotopes Ratio For compounds of chlorine and. An absolute value is obtained for the isotopic abundance ratio of bromine using thermal emission mass spectrometers calibrated for. Bromine isotopes refer to the two stable isotopes of bromine, 79br and 81br, with relative mass abundances of 50.686% and 49.314%. 49.5 if you want to be fussy!). This table shows information about naturally occuring isotopes,. Bromine Isotopes Ratio.
From www.chegg.com
Solved A scientist isolates a sample of bromine from an Bromine Isotopes Ratio For compounds of chlorine and. Isotope ratio of inorganic bromides was determined relative to isotope composition of standard mean ocean bromide (smob). All atomic nuclei of the chemical element bromine are summarized under. Bromine isotopes refer to the two stable isotopes of bromine, 79br and 81br, with relative mass abundances of 50.686% and 49.314%. Bromine has two isotopes, 79 br. Bromine Isotopes Ratio.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Isotopes Ratio This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. 49.5 if you want to be fussy!). Isotope ratio of inorganic bromides was determined relative to isotope composition of standard mean ocean bromide (smob). That means that a compound containing 1 bromine atom will have two peaks in. List,. Bromine Isotopes Ratio.
From www.researchgate.net
Relative variations of chlorine/bromine and carbon isotope ratios (Δ 37 Bromine Isotopes Ratio 49.5 if you want to be fussy!). That means that a compound containing 1 bromine atom will have two peaks in. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. All atomic. Bromine Isotopes Ratio.
From www.academia.edu
(PDF) High precision determination of bromine isotope ratio by GCMC Bromine Isotopes Ratio 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. Bromine isotopes refer to the two stable isotopes of bromine, 79br and 81br, with relative mass abundances of 50.686% and 49.314%. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : For compounds of chlorine. Bromine Isotopes Ratio.
From pubchem.ncbi.nlm.nih.gov
Bromine Br (Element) PubChem Bromine Isotopes Ratio For compounds of chlorine and. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. All atomic nuclei of the chemical element bromine are summarized under. Bromine isotopes refer to the two stable isotopes of. Bromine Isotopes Ratio.
From www.researchgate.net
Relative variations of chlorine/bromine and carbon isotope ratios (Δ 37 Bromine Isotopes Ratio This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. 49.5 if you want to be fussy!). That means that a compound containing 1 bromine atom will have two peaks. Bromine Isotopes Ratio.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron Bromine Isotopes Ratio That means that a compound containing 1 bromine atom will have two peaks in. Isotope ratio of inorganic bromides was determined relative to isotope composition of standard mean ocean bromide (smob). This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. 12 rows only the mass of the most. Bromine Isotopes Ratio.
From www.isotopes.gov
New domestic supply chain Bromine76 and Bromine77 NIDC National Bromine Isotopes Ratio For compounds of chlorine and. An absolute value is obtained for the isotopic abundance ratio of bromine using thermal emission mass spectrometers calibrated for. List, data and properties of all known isotopes of bromine. Bromine isotopes refer to the two stable isotopes of bromine, 79br and 81br, with relative mass abundances of 50.686% and 49.314%. 49.5 if you want to. Bromine Isotopes Ratio.
From www.numerade.com
SOLVEDNaturally occurring bromine consists of two isotopes, ^79 Br and Bromine Isotopes Ratio Bromine isotopes refer to the two stable isotopes of bromine, 79br and 81br, with relative mass abundances of 50.686% and 49.314%. All atomic nuclei of the chemical element bromine are summarized under. For compounds of chlorine and. 49.5 if you want to be fussy!). 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used. Bromine Isotopes Ratio.
From imgflip.com
Bromine Isotopic Abundance Imgflip Bromine Isotopes Ratio An absolute value is obtained for the isotopic abundance ratio of bromine using thermal emission mass spectrometers calibrated for. Isotope ratio of inorganic bromides was determined relative to isotope composition of standard mean ocean bromide (smob). 49.5 if you want to be fussy!). All atomic nuclei of the chemical element bromine are summarized under. That means that a compound containing. Bromine Isotopes Ratio.
From slideplayer.com
FH2O2Resonance [α]λ SN1E1 ME2SOCl2 TS Enan R. Chem Fall, ppt download Bromine Isotopes Ratio List, data and properties of all known isotopes of bromine. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : That means that a compound containing 1 bromine atom will have two peaks in. For compounds of chlorine and. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their. Bromine Isotopes Ratio.
From mavink.com
Lewis Dot Diagram For Bromine Bromine Isotopes Ratio An absolute value is obtained for the isotopic abundance ratio of bromine using thermal emission mass spectrometers calibrated for. Isotope ratio of inorganic bromides was determined relative to isotope composition of standard mean ocean bromide (smob). Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : List, data and properties of all known isotopes. Bromine Isotopes Ratio.
From www.numerade.com
SOLVED 'of 14 Give the nuclear symbol (isotope symbol) for the isotope Bromine Isotopes Ratio That means that a compound containing 1 bromine atom will have two peaks in. 12 rows only the mass of the most abundant isotope, relative to c (12.0000), is used for these calculations. Isotope ratio of inorganic bromides was determined relative to isotope composition of standard mean ocean bromide (smob). 49.5 if you want to be fussy!). Bromine has two. Bromine Isotopes Ratio.