Chlorine Molecule . chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. Find out its electronic configuration,. chlorine is a diatomic molecule and a halogen element with high electronegativity. It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. It is produced by electrolysis of seawater. It is essential to life as a chloride ion, and.
from cartoondealer.com
It is essential to life as a chloride ion, and. It is produced by electrolysis of seawater. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. Find out its electronic configuration,. chlorine is a diatomic molecule and a halogen element with high electronegativity.
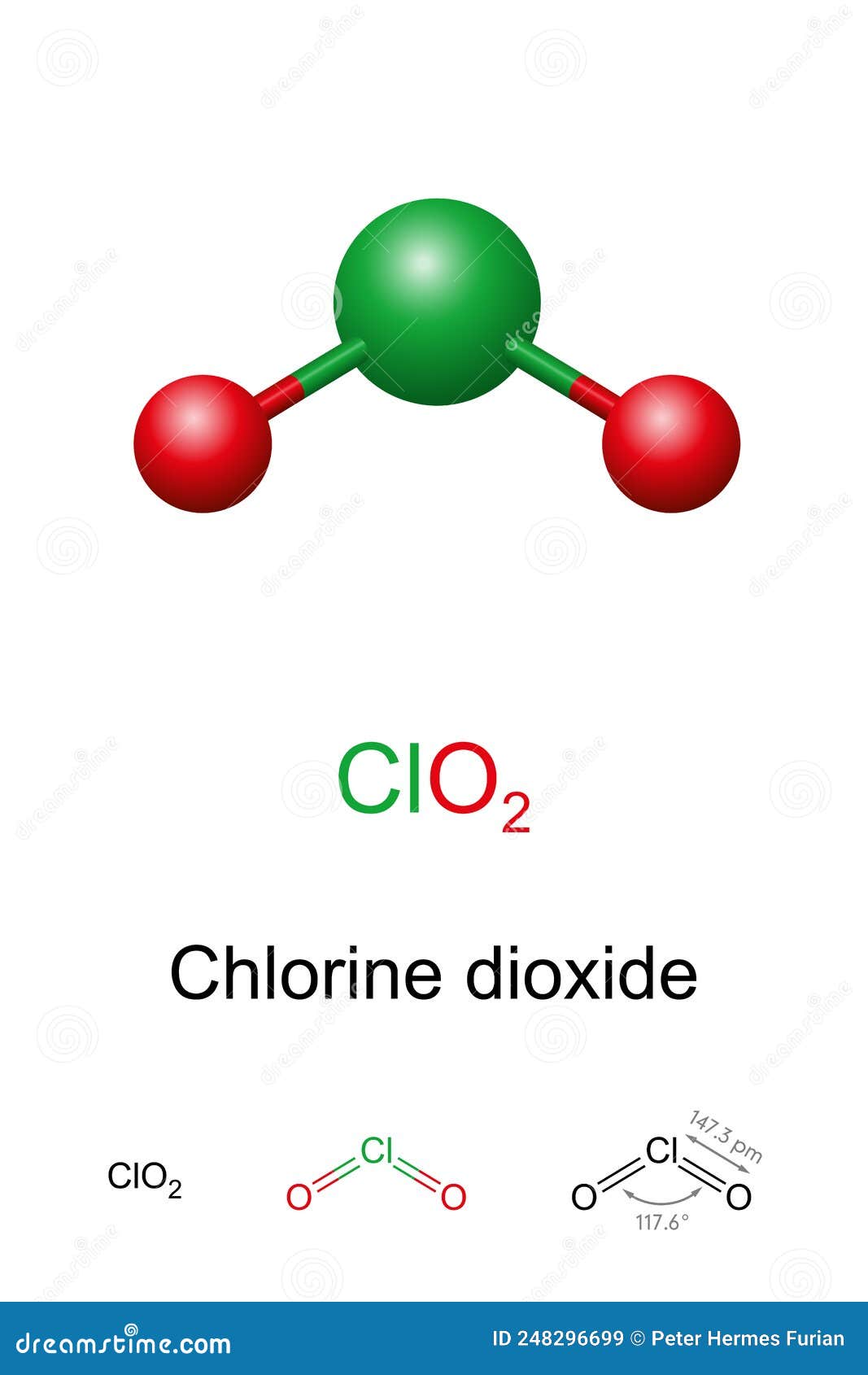
Chlorine Dioxide ClO2 Molecule. Used In Pulp Bleaching And For
Chlorine Molecule chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. chlorine is a diatomic molecule and a halogen element with high electronegativity. It is essential to life as a chloride ion, and. It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. Find out its electronic configuration,. chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. It is produced by electrolysis of seawater.
From
Chlorine Molecule — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. Find out its electronic configuration,. chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. chlorine is a diatomic molecule and a halogen element with. Chlorine Molecule.
From
Chlorine Molecule chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. chlorine is a diatomic molecule and a halogen element with high electronegativity. It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. Find out its electronic configuration,. . Chlorine Molecule.
From commons.wikimedia.org
FileChlorine3DvdW.png Chlorine Molecule chlorine is a diatomic molecule and a halogen element with high electronegativity. chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. Find out its electronic configuration,. chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety. Chlorine Molecule.
From
Chlorine Molecule chlorine is a diatomic molecule and a halogen element with high electronegativity. It is essential to life as a chloride ion, and. It is produced by electrolysis of seawater. chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. — chlorine is a greenish. Chlorine Molecule.
From
Chlorine Molecule chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. It is essential to life as a chloride ion, and. It has various applications in industry, medicine,. Chlorine Molecule.
From
Chlorine Molecule chlorine is a diatomic molecule and a halogen element with high electronegativity. It is essential to life as a chloride ion, and. chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. — chlorine is a greenish yellow gas composed of two atoms (cl2). Chlorine Molecule.
From
Chlorine Molecule Find out its electronic configuration,. chlorine is a diatomic molecule and a halogen element with high electronegativity. chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. It is produced. Chlorine Molecule.
From
Chlorine Molecule It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. Find out its electronic configuration,. chlorine is a diatomic molecule and a halogen element with high electronegativity. chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. chlorine, as chlorine gas, chlorite. Chlorine Molecule.
From www.alamy.com
Chlorine element hires stock photography and images Alamy Chlorine Molecule It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. It is essential to life as a chloride ion, and. Find out its electronic configuration,. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. It is produced by electrolysis of seawater. . Chlorine Molecule.
From
Chlorine Molecule chlorine is a diatomic molecule and a halogen element with high electronegativity. It is produced by electrolysis of seawater. It is essential to life as a chloride ion, and. Find out its electronic configuration,. It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. chlorine is a diatomic gas belonging to. Chlorine Molecule.
From
Chlorine Molecule chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. It is essential to life as a chloride ion, and. It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. — chlorine is a greenish yellow gas composed. Chlorine Molecule.
From
Chlorine Molecule It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. It is produced by electrolysis of seawater. It is essential to life as a chloride ion, and. chlorine is a diatomic gas. Chlorine Molecule.
From
Chlorine Molecule It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. It is produced by electrolysis of seawater. chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. chlorine is a diatomic molecule and a halogen element with high electronegativity. It is essential to. Chlorine Molecule.
From
Chlorine Molecule chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. chlorine is a diatomic molecule and a halogen element with high electronegativity. It is produced by electrolysis of seawater. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with. Chlorine Molecule.
From
Chlorine Molecule chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. It has various applications in industry, medicine, and household products, but it is also toxic and. Chlorine Molecule.
From
Chlorine Molecule chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and. Chlorine Molecule.
From fineartamerica.com
Bond Formation In Chlorine Molecule Photograph by Chlorine Molecule chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. chlorine is a diatomic molecule and a halogen element with high electronegativity. It is produced by electrolysis of seawater. chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce. Chlorine Molecule.
From
Chlorine Molecule It is produced by electrolysis of seawater. It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. It is essential to life as a chloride ion, and. Find out its electronic configuration,. . Chlorine Molecule.
From www.alamy.com
Chlorine molecule. Computer illustration showing the structure of a Chlorine Molecule It is essential to life as a chloride ion, and. It is produced by electrolysis of seawater. chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. chlorine is a diatomic molecule and a halogen element with high electronegativity. Find out its electronic configuration,. It has various applications in industry,. Chlorine Molecule.
From
Chlorine Molecule chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. It is produced by electrolysis of seawater. Find out its electronic configuration,. — chlorine is a greenish yellow. Chlorine Molecule.
From
Chlorine Molecule Find out its electronic configuration,. It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. It is essential to life as a chloride ion, and. It is produced by electrolysis of seawater. . Chlorine Molecule.
From
Chlorine Molecule It is essential to life as a chloride ion, and. chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. chlorine is a diatomic molecule and a halogen element with high electronegativity. chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic. Chlorine Molecule.
From
Chlorine Molecule It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. chlorine is a diatomic molecule and a halogen element with high electronegativity. — chlorine is a greenish. Chlorine Molecule.
From
Chlorine Molecule chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. Find out its electronic configuration,. It is essential to life as a chloride ion, and. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. It has various applications in industry,. Chlorine Molecule.
From www.vectorstock.com
Cl2 chlorine molecule Royalty Free Vector Image Chlorine Molecule It is produced by electrolysis of seawater. chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. It is essential to life as a chloride ion, and. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and. Chlorine Molecule.
From
Chlorine Molecule It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. It is produced by electrolysis of seawater. chlorine is a diatomic gas belonging to the halogen family with the symbol cl and. Chlorine Molecule.
From
Chlorine Molecule chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many. Chlorine Molecule.
From
Chlorine Molecule — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. It is essential to life as a chloride ion, and. Find out its electronic configuration,. chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. It has various applications in industry,. Chlorine Molecule.
From www.vectorstock.com
Atom chlorine Royalty Free Vector Image VectorStock Chlorine Molecule Find out its electronic configuration,. It is produced by electrolysis of seawater. It is essential to life as a chloride ion, and. chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. chlorine. Chlorine Molecule.
From
Chlorine Molecule chlorine is a diatomic molecule and a halogen element with high electronegativity. chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. chlorine is a diatomic gas. Chlorine Molecule.
From
Chlorine Molecule chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce a variety of. It is essential to life as a chloride ion, and. chlorine is a diatomic molecule and a halogen element with high electronegativity. chlorine is a diatomic gas belonging to the halogen family with the. Chlorine Molecule.
From
Chlorine Molecule Find out its electronic configuration,. It is produced by electrolysis of seawater. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. It has various applications in industry, medicine, and household. Chlorine Molecule.
From
Chlorine Molecule It is produced by electrolysis of seawater. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. chlorine is a diatomic molecule and a halogen element with high electronegativity. Find out its electronic configuration,. It is essential to life as a chloride ion, and. It has various applications in. Chlorine Molecule.
From
Chlorine Molecule chlorine is a diatomic gas belonging to the halogen family with the symbol cl and atomic number 17. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds. chlorine, as chlorine gas, chlorite ion, and hypochlorite, is a strong oxidant that readily reacts with organic molecules to produce. Chlorine Molecule.
From
Chlorine Molecule chlorine is a diatomic molecule and a halogen element with high electronegativity. It is essential to life as a chloride ion, and. It has various applications in industry, medicine, and household products, but it is also toxic and corrosive. — chlorine is a greenish yellow gas composed of two atoms (cl2) that reacts with many elements and compounds.. Chlorine Molecule.