Electrochemistry Exercises And Answers . electrochemistry practice problems include questions on calculating eo and e, δg based on the cell potential, nernst equation, and more. Key equations given for test: Al (s) or zn (s) or diatomics) 2. Click the card to flip 👆. a multiple choice practice quiz on electrochemistry to practice galvanic and voltaic cells, nernst equation, cell. the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. what are the rules for oxidation numbers? Free state = 0 (ex: explain how rusting of iron is envisaged as setting up of an electrochemical cell. these are homework exercises to accompany the textmap created for chemistry: What is the oxidation number of chromium.
from www.liveworksheets.com
Key equations given for test: electrochemistry practice problems include questions on calculating eo and e, δg based on the cell potential, nernst equation, and more. Click the card to flip 👆. Al (s) or zn (s) or diatomics) 2. these are homework exercises to accompany the textmap created for chemistry: Free state = 0 (ex: explain how rusting of iron is envisaged as setting up of an electrochemical cell. What is the oxidation number of chromium. what are the rules for oxidation numbers? the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \.
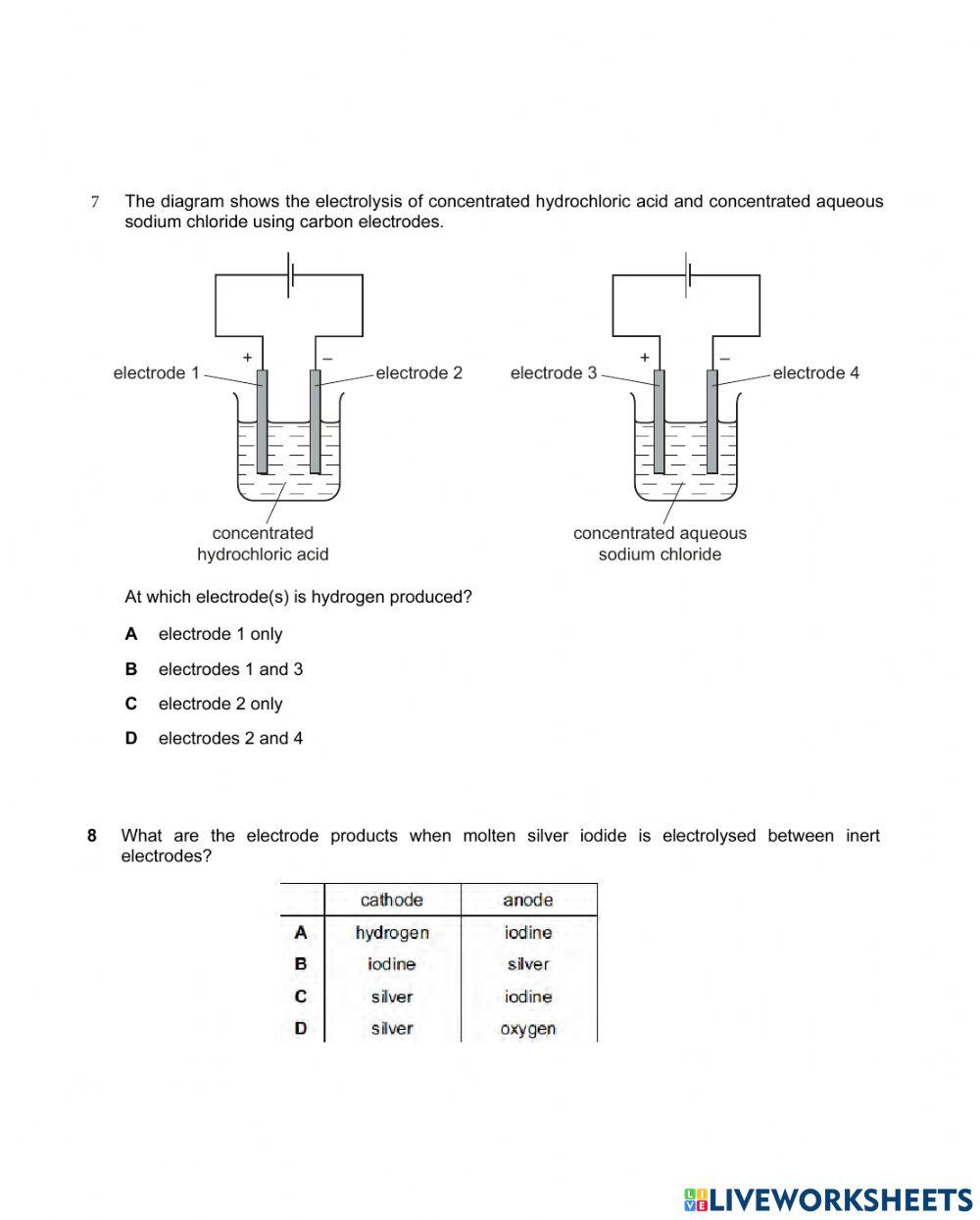
Electrochemistry 9 IGCSE AND 10 IGCSE online exercise for Live Worksheets
Electrochemistry Exercises And Answers Click the card to flip 👆. a multiple choice practice quiz on electrochemistry to practice galvanic and voltaic cells, nernst equation, cell. what are the rules for oxidation numbers? electrochemistry practice problems include questions on calculating eo and e, δg based on the cell potential, nernst equation, and more. Free state = 0 (ex: these are homework exercises to accompany the textmap created for chemistry: What is the oxidation number of chromium. Al (s) or zn (s) or diatomics) 2. Key equations given for test: the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. explain how rusting of iron is envisaged as setting up of an electrochemical cell. Click the card to flip 👆.
From adamjeecoaching.blogspot.com
Adamjee Coaching Electrochemistry Short and Detailed Question Electrochemistry Exercises And Answers Free state = 0 (ex: Al (s) or zn (s) or diatomics) 2. What is the oxidation number of chromium. Key equations given for test: what are the rules for oxidation numbers? a multiple choice practice quiz on electrochemistry to practice galvanic and voltaic cells, nernst equation, cell. explain how rusting of iron is envisaged as setting. Electrochemistry Exercises And Answers.
From www.studocu.com
Exercises Electrochemistry with answer Physical Chemistry Studocu Electrochemistry Exercises And Answers Al (s) or zn (s) or diatomics) 2. What is the oxidation number of chromium. explain how rusting of iron is envisaged as setting up of an electrochemical cell. Click the card to flip 👆. electrochemistry practice problems include questions on calculating eo and e, δg based on the cell potential, nernst equation, and more. the overall. Electrochemistry Exercises And Answers.
From studylib.net
Honors Chemistry Electrochemistry Worksheet Answers 1. A 2. B 3 Electrochemistry Exercises And Answers Al (s) or zn (s) or diatomics) 2. what are the rules for oxidation numbers? the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. Free state = 0 (ex: electrochemistry practice problems include questions on calculating eo and e, δg based on the. Electrochemistry Exercises And Answers.
From www.studocu.com
PRP1026 LU7 Electrochemistry Additional Exercises (Lectures 1 3 Electrochemistry Exercises And Answers a multiple choice practice quiz on electrochemistry to practice galvanic and voltaic cells, nernst equation, cell. what are the rules for oxidation numbers? Free state = 0 (ex: the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. these are homework exercises to. Electrochemistry Exercises And Answers.
From www.chegg.com
Solved Electrochemistry Exercises And Answers Click the card to flip 👆. electrochemistry practice problems include questions on calculating eo and e, δg based on the cell potential, nernst equation, and more. the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. Free state = 0 (ex: what are the. Electrochemistry Exercises And Answers.
From chemistry.analia-sanchez.net
Electrochemistry Exercises 2 Balancing redox equations Chemistry Electrochemistry Exercises And Answers Key equations given for test: What is the oxidation number of chromium. what are the rules for oxidation numbers? Al (s) or zn (s) or diatomics) 2. the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. Click the card to flip 👆. explain. Electrochemistry Exercises And Answers.
From www.chegg.com
Solved Thermodynamics & Electrochemistry 00 8314 Exercises Electrochemistry Exercises And Answers Free state = 0 (ex: Key equations given for test: What is the oxidation number of chromium. Al (s) or zn (s) or diatomics) 2. the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. electrochemistry practice problems include questions on calculating eo and e,. Electrochemistry Exercises And Answers.
From chem.libretexts.org
17.E Electrochemistry (Exercises) Chemistry LibreTexts Electrochemistry Exercises And Answers Key equations given for test: Click the card to flip 👆. What is the oxidation number of chromium. these are homework exercises to accompany the textmap created for chemistry: Free state = 0 (ex: what are the rules for oxidation numbers? the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+}. Electrochemistry Exercises And Answers.
From www.scribd.com
Electrochemistry Exercises Solutions PDF Electrochemistry Electrochemistry Exercises And Answers these are homework exercises to accompany the textmap created for chemistry: the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. Key equations given for test: Click the card to flip 👆. What is the oxidation number of chromium. what are the rules for. Electrochemistry Exercises And Answers.
From www.ecolebooks.com
21. Electrochemistry Questions and Answers EcoleBooks Electrochemistry Exercises And Answers explain how rusting of iron is envisaged as setting up of an electrochemical cell. Click the card to flip 👆. these are homework exercises to accompany the textmap created for chemistry: what are the rules for oxidation numbers? Al (s) or zn (s) or diatomics) 2. Free state = 0 (ex: What is the oxidation number of. Electrochemistry Exercises And Answers.
From www.chegg.com
Solved Material electrochemical exercises Electrochemistry Exercises And Answers Free state = 0 (ex: explain how rusting of iron is envisaged as setting up of an electrochemical cell. what are the rules for oxidation numbers? Al (s) or zn (s) or diatomics) 2. Click the card to flip 👆. electrochemistry practice problems include questions on calculating eo and e, δg based on the cell potential, nernst. Electrochemistry Exercises And Answers.
From studylib.net
Electrochemistry Worksheet Electrochemistry Exercises And Answers the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. these are homework exercises to accompany the textmap created for chemistry: a multiple choice practice quiz on electrochemistry to practice galvanic and voltaic cells, nernst equation, cell. electrochemistry practice problems include questions on. Electrochemistry Exercises And Answers.
From www.scribd.com
Chem 1B Chapter 18 Exercises With Answers PDF Redox Electrochemistry Electrochemistry Exercises And Answers Click the card to flip 👆. what are the rules for oxidation numbers? Al (s) or zn (s) or diatomics) 2. a multiple choice practice quiz on electrochemistry to practice galvanic and voltaic cells, nernst equation, cell. Free state = 0 (ex: explain how rusting of iron is envisaged as setting up of an electrochemical cell. What. Electrochemistry Exercises And Answers.
From www.scribd.com
Unit 2 Electrochemistry Practice Exercises Chemistry For Engineers Electrochemistry Exercises And Answers what are the rules for oxidation numbers? Free state = 0 (ex: Click the card to flip 👆. What is the oxidation number of chromium. Key equations given for test: Al (s) or zn (s) or diatomics) 2. a multiple choice practice quiz on electrochemistry to practice galvanic and voltaic cells, nernst equation, cell. explain how rusting. Electrochemistry Exercises And Answers.
From dokumen.tips
(PDF) 20. Electrochemistry Exercises And Answers the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. Free state = 0 (ex: explain how rusting of iron is envisaged as setting up of an electrochemical cell. Click the card to flip 👆. electrochemistry practice problems include questions on calculating eo and. Electrochemistry Exercises And Answers.
From www.youtube.com
11th Chemistry, Chapter 10, Electrochemistry, Solved Exercise Questions Electrochemistry Exercises And Answers Key equations given for test: Free state = 0 (ex: these are homework exercises to accompany the textmap created for chemistry: what are the rules for oxidation numbers? Click the card to flip 👆. Al (s) or zn (s) or diatomics) 2. explain how rusting of iron is envisaged as setting up of an electrochemical cell. What. Electrochemistry Exercises And Answers.
From byjus.com
Class 12 Chemistry Worksheet on Chapter 3 Electrochemistry Set 2 Electrochemistry Exercises And Answers Click the card to flip 👆. explain how rusting of iron is envisaged as setting up of an electrochemical cell. these are homework exercises to accompany the textmap created for chemistry: Free state = 0 (ex: a multiple choice practice quiz on electrochemistry to practice galvanic and voltaic cells, nernst equation, cell. electrochemistry practice problems include. Electrochemistry Exercises And Answers.
From www.chegg.com
Solved Material electrochemical exercises Electrochemistry Exercises And Answers Click the card to flip 👆. what are the rules for oxidation numbers? a multiple choice practice quiz on electrochemistry to practice galvanic and voltaic cells, nernst equation, cell. these are homework exercises to accompany the textmap created for chemistry: explain how rusting of iron is envisaged as setting up of an electrochemical cell. electrochemistry. Electrochemistry Exercises And Answers.
From pdfprof.com
electrochemistry exercises and answers Electrochemistry Exercises And Answers a multiple choice practice quiz on electrochemistry to practice galvanic and voltaic cells, nernst equation, cell. these are homework exercises to accompany the textmap created for chemistry: What is the oxidation number of chromium. the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \.. Electrochemistry Exercises And Answers.
From www.pdfprof.com
electrochemistry exam questions answers Electrochemistry Exercises And Answers a multiple choice practice quiz on electrochemistry to practice galvanic and voltaic cells, nernst equation, cell. the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. explain how rusting of iron is envisaged as setting up of an electrochemical cell. these are homework. Electrochemistry Exercises And Answers.
From adamjeecoaching.blogspot.com
Adamjee Coaching Electrochemistry Short and Detailed Question Electrochemistry Exercises And Answers the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. electrochemistry practice problems include questions on calculating eo and e, δg based on the cell potential, nernst equation, and more. what are the rules for oxidation numbers? Al (s) or zn (s) or diatomics). Electrochemistry Exercises And Answers.
From www.scribd.com
25 Redox Reactions Practice Worksheet With Answers Redox Electrochemistry Exercises And Answers explain how rusting of iron is envisaged as setting up of an electrochemical cell. What is the oxidation number of chromium. Free state = 0 (ex: the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. electrochemistry practice problems include questions on calculating eo. Electrochemistry Exercises And Answers.
From padhainotes.com
Class 12 Chemistry Chapter 3 Electrochemistry Question Answer Padhai Electrochemistry Exercises And Answers the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. Key equations given for test: explain how rusting of iron is envisaged as setting up of an electrochemical cell. Free state = 0 (ex: What is the oxidation number of chromium. these are homework. Electrochemistry Exercises And Answers.
From byjus.com
Class 12 Chemistry Worksheet on Chapter 3 Electrochemistry Set 4 Electrochemistry Exercises And Answers explain how rusting of iron is envisaged as setting up of an electrochemical cell. what are the rules for oxidation numbers? a multiple choice practice quiz on electrochemistry to practice galvanic and voltaic cells, nernst equation, cell. these are homework exercises to accompany the textmap created for chemistry: Al (s) or zn (s) or diatomics) 2.. Electrochemistry Exercises And Answers.
From www.studocu.com
Session 18 Electrochemistry MC Answer Key ElectrochemistryCh Electrochemistry Exercises And Answers a multiple choice practice quiz on electrochemistry to practice galvanic and voltaic cells, nernst equation, cell. Key equations given for test: what are the rules for oxidation numbers? Al (s) or zn (s) or diatomics) 2. What is the oxidation number of chromium. Click the card to flip 👆. these are homework exercises to accompany the textmap. Electrochemistry Exercises And Answers.
From pdfprof.com
electrochemistry worksheet with answers pdf Electrochemistry Exercises And Answers these are homework exercises to accompany the textmap created for chemistry: Free state = 0 (ex: Click the card to flip 👆. Key equations given for test: electrochemistry practice problems include questions on calculating eo and e, δg based on the cell potential, nernst equation, and more. What is the oxidation number of chromium. a multiple choice. Electrochemistry Exercises And Answers.
From www.chegg.com
Solved Material electrochemical exercises Electrochemistry Exercises And Answers electrochemistry practice problems include questions on calculating eo and e, δg based on the cell potential, nernst equation, and more. explain how rusting of iron is envisaged as setting up of an electrochemical cell. Free state = 0 (ex: Al (s) or zn (s) or diatomics) 2. what are the rules for oxidation numbers? a multiple. Electrochemistry Exercises And Answers.
From www.studocu.com
Electrochemistry practise questions answers CHEM 3 Practise Electrochemistry Exercises And Answers what are the rules for oxidation numbers? What is the oxidation number of chromium. Click the card to flip 👆. Al (s) or zn (s) or diatomics) 2. these are homework exercises to accompany the textmap created for chemistry: explain how rusting of iron is envisaged as setting up of an electrochemical cell. a multiple choice. Electrochemistry Exercises And Answers.
From www.liveworksheets.com
Electrochemistry 9 IGCSE AND 10 IGCSE online exercise for Live Worksheets Electrochemistry Exercises And Answers Click the card to flip 👆. the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. electrochemistry practice problems include questions on calculating eo and e, δg based on the cell potential, nernst equation, and more. these are homework exercises to accompany the textmap. Electrochemistry Exercises And Answers.
From studylib.net
Electrochemistry Exercises Electrochemistry Exercises And Answers Click the card to flip 👆. what are the rules for oxidation numbers? these are homework exercises to accompany the textmap created for chemistry: Key equations given for test: Free state = 0 (ex: What is the oxidation number of chromium. electrochemistry practice problems include questions on calculating eo and e, δg based on the cell potential,. Electrochemistry Exercises And Answers.
From www.scribd.com
Question and answer on electrochemistry.pdf Redox Electrochemistry Electrochemistry Exercises And Answers Al (s) or zn (s) or diatomics) 2. a multiple choice practice quiz on electrochemistry to practice galvanic and voltaic cells, nernst equation, cell. Click the card to flip 👆. these are homework exercises to accompany the textmap created for chemistry: Key equations given for test: electrochemistry practice problems include questions on calculating eo and e, δg. Electrochemistry Exercises And Answers.
From www.chegg.com
Solved Material electrochemical exercises Electrochemistry Exercises And Answers Al (s) or zn (s) or diatomics) 2. Click the card to flip 👆. electrochemistry practice problems include questions on calculating eo and e, δg based on the cell potential, nernst equation, and more. these are homework exercises to accompany the textmap created for chemistry: a multiple choice practice quiz on electrochemistry to practice galvanic and voltaic. Electrochemistry Exercises And Answers.
From www.studypool.com
SOLUTION Exercises on electrochemistry grade 12 level Studypool Electrochemistry Exercises And Answers Click the card to flip 👆. the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. explain how rusting of iron is envisaged as setting up of an electrochemical cell. Key equations given for test: these are homework exercises to accompany the textmap created. Electrochemistry Exercises And Answers.
From www.youtube.com
11th Chemistry, Chapter 10, Electrochemistry, Solved Exercise Questions Electrochemistry Exercises And Answers what are the rules for oxidation numbers? the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. Click the card to flip 👆. What is the oxidation number of chromium. Free state = 0 (ex: a multiple choice practice quiz on electrochemistry to practice. Electrochemistry Exercises And Answers.
From www.youtube.com
11th Chemistry, Chapter 10, Electrochemistry, Solved Exercise Questions Electrochemistry Exercises And Answers what are the rules for oxidation numbers? the overall equation would be \ (\mathrm {pb^ {2+} (aq) + sn (s) \rightarrow sn^ {2+} (aq) + pb (s)}\) thus, the \. Al (s) or zn (s) or diatomics) 2. What is the oxidation number of chromium. Key equations given for test: Click the card to flip 👆. Free state. Electrochemistry Exercises And Answers.