Mdd Mdr Gap Analysis . You can download it free, fill it out and if you want send it back to us,. Mdr gap assessment tool with comparison table of standard requirements for medical devices to assess correlations, identify gaps and streamline. Truly the best resource is bsi transition to mdr page. This tool will help focusing the requirement introduced by the new mdr. Eu mdd to mdr gap analysis for medical device ce marking. The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. An mdr gap analysis is the process of systematically examining a medical device’s clinical evidence portfolio to determine whether it demonstrates conformity with. Ce mdr gap analysis with emergo by ul. Specifically, i recommend the following: Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to be prepared in order to transition to the mdr.
from intellisoft.io
Mdr gap assessment tool with comparison table of standard requirements for medical devices to assess correlations, identify gaps and streamline. Eu mdd to mdr gap analysis for medical device ce marking. Ce mdr gap analysis with emergo by ul. The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to be prepared in order to transition to the mdr. Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. Truly the best resource is bsi transition to mdr page. This tool will help focusing the requirement introduced by the new mdr. Specifically, i recommend the following: An mdr gap analysis is the process of systematically examining a medical device’s clinical evidence portfolio to determine whether it demonstrates conformity with.
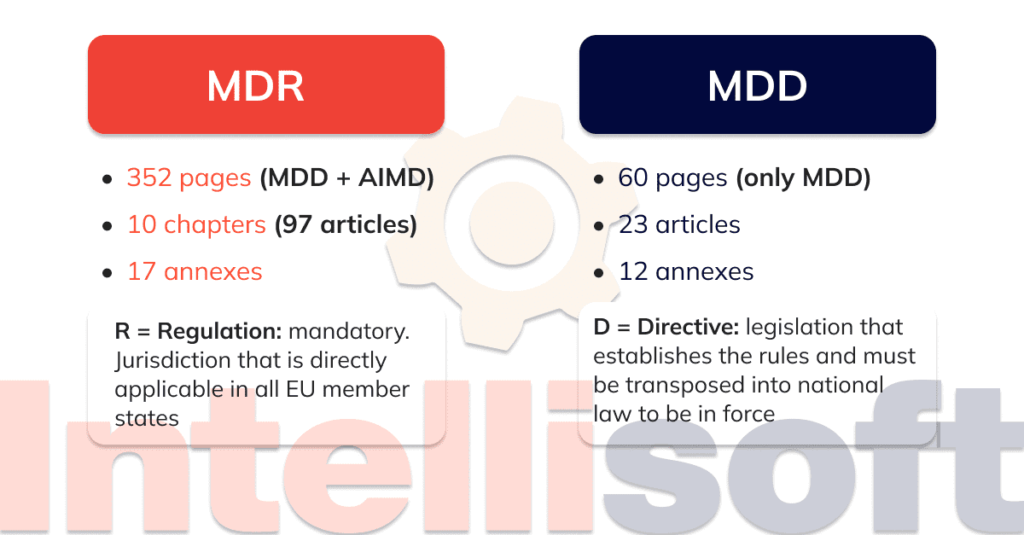
EU Regulation Transitioning from the MDD to MDR
Mdd Mdr Gap Analysis Eu mdd to mdr gap analysis for medical device ce marking. Mdr gap assessment tool with comparison table of standard requirements for medical devices to assess correlations, identify gaps and streamline. Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. Ce mdr gap analysis with emergo by ul. An mdr gap analysis is the process of systematically examining a medical device’s clinical evidence portfolio to determine whether it demonstrates conformity with. The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to be prepared in order to transition to the mdr. You can download it free, fill it out and if you want send it back to us,. Truly the best resource is bsi transition to mdr page. This tool will help focusing the requirement introduced by the new mdr. Specifically, i recommend the following: Eu mdd to mdr gap analysis for medical device ce marking.
From swarali-bhakre.medium.com
What is an MDR Gap Analysis?. Until today, medical devices were… by Mdd Mdr Gap Analysis You can download it free, fill it out and if you want send it back to us,. Ce mdr gap analysis with emergo by ul. Mdr gap assessment tool with comparison table of standard requirements for medical devices to assess correlations, identify gaps and streamline. Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. This tool. Mdd Mdr Gap Analysis.
From mavenprofserv.com
Gap Analysis MDD to EUMDR & IVDD to IVDR Mdd Mdr Gap Analysis Mdr gap assessment tool with comparison table of standard requirements for medical devices to assess correlations, identify gaps and streamline. An mdr gap analysis is the process of systematically examining a medical device’s clinical evidence portfolio to determine whether it demonstrates conformity with. The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Truly the best resource is bsi. Mdd Mdr Gap Analysis.
From tsquality.ch
MDR vs MDD QUICK COMPARISON TSQuality.ch Mdd Mdr Gap Analysis Specifically, i recommend the following: The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Ce mdr gap analysis with emergo by ul. This tool will help focusing the requirement introduced by the new mdr. Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. Manufacturers with devices on the market under the mdd should perform. Mdd Mdr Gap Analysis.
From www.researchgate.net
Overview of the Conceptual Gap Analysis of CDD and MDD tools method Mdd Mdr Gap Analysis You can download it free, fill it out and if you want send it back to us,. This tool will help focusing the requirement introduced by the new mdr. Ce mdr gap analysis with emergo by ul. Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. An mdr gap analysis is the process of systematically examining. Mdd Mdr Gap Analysis.
From www.greenlight.guru
MDD vs. MDR Gap Assessment Tool Free Download Mdd Mdr Gap Analysis Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to be prepared in order to transition to the mdr. An mdr gap analysis is the. Mdd Mdr Gap Analysis.
From www.regulatoryglobe.com
MDR GapAssessment Tool incl. ISO 134852016 Mdd Mdr Gap Analysis Ce mdr gap analysis with emergo by ul. You can download it free, fill it out and if you want send it back to us,. Mdr gap assessment tool with comparison table of standard requirements for medical devices to assess correlations, identify gaps and streamline. Specifically, i recommend the following: Truly the best resource is bsi transition to mdr page.. Mdd Mdr Gap Analysis.
From www.scribd.com
Methodology of Using The MDD vs. MDR Gap Assessment Tool Distributed Mdd Mdr Gap Analysis This tool will help focusing the requirement introduced by the new mdr. Ce mdr gap analysis with emergo by ul. The eu medical device regulation 2017/745 (eu mdr) replaces the medical. You can download it free, fill it out and if you want send it back to us,. Eu mdd to mdr gap analysis for medical device ce marking. Manufacturers. Mdd Mdr Gap Analysis.
From www.slideegg.com
MDD To MDR Gap Analysis Template PowerPoint & Google Slides Mdd Mdr Gap Analysis Ce mdr gap analysis with emergo by ul. Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. Eu mdd to mdr gap analysis for medical device ce marking. Truly the best resource is bsi transition to mdr page. Specifically, i recommend the following: An mdr gap analysis is the process of systematically examining a medical device’s. Mdd Mdr Gap Analysis.
From www.linkedin.com
How to Perform an MDR Gap Analysis Mdd Mdr Gap Analysis Eu mdd to mdr gap analysis for medical device ce marking. Truly the best resource is bsi transition to mdr page. Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to be prepared in order to transition to the mdr. Ce mdr gap analysis with emergo by ul. Specifically,. Mdd Mdr Gap Analysis.
From www.greenlight.guru
MDR Gap Analysis Tool Greenlight Guru Mdd Mdr Gap Analysis This tool will help focusing the requirement introduced by the new mdr. Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to be prepared in order to transition to the mdr. The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Specifically, i recommend the following: Truly the. Mdd Mdr Gap Analysis.
From tsquality.ch
MDR vs MDD QUICK COMPARISON TSQuality.ch Mdd Mdr Gap Analysis An mdr gap analysis is the process of systematically examining a medical device’s clinical evidence portfolio to determine whether it demonstrates conformity with. Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Mdr gap assessment tool with comparison table of standard requirements for medical devices. Mdd Mdr Gap Analysis.
From www.greenlight.guru
MDD vs. MDR Gap Assessment Tool Greenlight Guru Mdd Mdr Gap Analysis Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to be prepared in order to transition to the mdr. Eu mdd to mdr gap analysis for medical device ce marking. An mdr gap analysis is the process of systematically examining a medical device’s clinical evidence portfolio to determine whether. Mdd Mdr Gap Analysis.
From tsquality.ch
MDR vs MDD QUICK COMPARISON TSQuality.ch Mdd Mdr Gap Analysis Mdr gap assessment tool with comparison table of standard requirements for medical devices to assess correlations, identify gaps and streamline. Ce mdr gap analysis with emergo by ul. The eu medical device regulation 2017/745 (eu mdr) replaces the medical. An mdr gap analysis is the process of systematically examining a medical device’s clinical evidence portfolio to determine whether it demonstrates. Mdd Mdr Gap Analysis.
From mavenprofserv.com
Gap Analysis MDD to EUMDR & IVDD to IVDR Mdd Mdr Gap Analysis Eu mdd to mdr gap analysis for medical device ce marking. You can download it free, fill it out and if you want send it back to us,. Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to be prepared in order to transition to the mdr. Specifically, i. Mdd Mdr Gap Analysis.
From mavenprofserv.com
Gap Analysis MDD to EUMDR & IVDD to IVDR Mdd Mdr Gap Analysis Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to be prepared in order to transition to the mdr. The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Mdr gap assessment tool with comparison table of standard requirements for medical devices to assess correlations, identify gaps and. Mdd Mdr Gap Analysis.
From www.i3cglobal.com
MDR Technical File GAP Analysis Checklist I3CGlobal Mdd Mdr Gap Analysis Truly the best resource is bsi transition to mdr page. Eu mdd to mdr gap analysis for medical device ce marking. Specifically, i recommend the following: Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to. Mdd Mdr Gap Analysis.
From www.scribd.com
Methodology of Using The MDD vs. MDR Gap Assessment Tool FreeVer Mdd Mdr Gap Analysis This tool will help focusing the requirement introduced by the new mdr. Eu mdd to mdr gap analysis for medical device ce marking. Truly the best resource is bsi transition to mdr page. Specifically, i recommend the following: Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. Mdr gap assessment tool with comparison table of standard. Mdd Mdr Gap Analysis.
From www.mdr.guide
MDR Guidance Medical Device Regulatory Guide Mdd Mdr Gap Analysis Ce mdr gap analysis with emergo by ul. Truly the best resource is bsi transition to mdr page. The eu medical device regulation 2017/745 (eu mdr) replaces the medical. You can download it free, fill it out and if you want send it back to us,. This tool will help focusing the requirement introduced by the new mdr. Eu mdd. Mdd Mdr Gap Analysis.
From www.youtube.com
Gap Analysis (MDD to MDR, IVDD to IVDR, QMS, Standards) YouTube Mdd Mdr Gap Analysis The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Specifically, i recommend the following: Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. Eu mdd to mdr gap analysis for medical device ce marking. You can download it free, fill it out and if you want send it back to us,. Mdr gap assessment. Mdd Mdr Gap Analysis.
From www.mantrasystems.co.uk
An MDR Gap Analysis Summary Mdd Mdr Gap Analysis Ce mdr gap analysis with emergo by ul. Specifically, i recommend the following: Mdr gap assessment tool with comparison table of standard requirements for medical devices to assess correlations, identify gaps and streamline. Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to be prepared in order to transition. Mdd Mdr Gap Analysis.
From www.thefdagroup.com
MDR vs. MDD 13 Key Changes Mdd Mdr Gap Analysis An mdr gap analysis is the process of systematically examining a medical device’s clinical evidence portfolio to determine whether it demonstrates conformity with. This tool will help focusing the requirement introduced by the new mdr. Truly the best resource is bsi transition to mdr page. Mdr gap assessment tool with comparison table of standard requirements for medical devices to assess. Mdd Mdr Gap Analysis.
From mavenprofserv.com
Gap Analysis MDD to EUMDR & IVDD to IVDR Mdd Mdr Gap Analysis Eu mdd to mdr gap analysis for medical device ce marking. Mdr gap assessment tool with comparison table of standard requirements for medical devices to assess correlations, identify gaps and streamline. Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. An mdr gap analysis is the process of systematically examining a medical device’s clinical evidence portfolio. Mdd Mdr Gap Analysis.
From tsquality.ch
MDR vs MDD QUICK COMPARISON TSQuality.ch Mdd Mdr Gap Analysis Mdr gap assessment tool with comparison table of standard requirements for medical devices to assess correlations, identify gaps and streamline. Eu mdd to mdr gap analysis for medical device ce marking. Specifically, i recommend the following: The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Manufacturers with devices on the market under the mdd should perform a gap. Mdd Mdr Gap Analysis.
From www.slideserve.com
PPT What is an MDR Gap Analysis PowerPoint Presentation, free Mdd Mdr Gap Analysis This tool will help focusing the requirement introduced by the new mdr. An mdr gap analysis is the process of systematically examining a medical device’s clinical evidence portfolio to determine whether it demonstrates conformity with. Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to be prepared in order. Mdd Mdr Gap Analysis.
From www.greenlight.guru
MDD vs. MDR Gap Assessment Tool Free Download Mdd Mdr Gap Analysis Eu mdd to mdr gap analysis for medical device ce marking. Ce mdr gap analysis with emergo by ul. You can download it free, fill it out and if you want send it back to us,. Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. Truly the best resource is bsi transition to mdr page. An. Mdd Mdr Gap Analysis.
From www.regulatoryglobe.com
MDD vs MDR GapAssessment Tool Mdd Mdr Gap Analysis Eu mdd to mdr gap analysis for medical device ce marking. You can download it free, fill it out and if you want send it back to us,. Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Specifically, i recommend the following: An mdr gap. Mdd Mdr Gap Analysis.
From www.scribd.com
MDD_vs_MDR_Gap_Assessment_Tool_2017_745Distributed_By_Greenlight_Guru Mdd Mdr Gap Analysis The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Emergo can perform a systematic, independent gap analysis of your ce technical documentation,. This tool will help focusing the requirement introduced by the new mdr. Ce mdr gap analysis with emergo by ul. Truly the best resource is bsi transition to mdr page. An mdr gap analysis is the. Mdd Mdr Gap Analysis.
From mlreoconsulting.com
MDR Compliance Gap Analysis Spreadsheet [format *.xlsx] M.L. Reo Mdd Mdr Gap Analysis Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to be prepared in order to transition to the mdr. You can download it free, fill it out and if you want send it back to us,. Emergo can perform a systematic, independent gap analysis of your ce technical documentation,.. Mdd Mdr Gap Analysis.
From gidos.net
Gap Assessment MDD GiDoS Mdd Mdr Gap Analysis An mdr gap analysis is the process of systematically examining a medical device’s clinical evidence portfolio to determine whether it demonstrates conformity with. Eu mdd to mdr gap analysis for medical device ce marking. The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Specifically, i recommend the following: You can download it free, fill it out and if. Mdd Mdr Gap Analysis.
From mavenprofserv.com
Gap Analysis MDD to EUMDR & IVDD to IVDR Mdd Mdr Gap Analysis Truly the best resource is bsi transition to mdr page. Ce mdr gap analysis with emergo by ul. You can download it free, fill it out and if you want send it back to us,. Eu mdd to mdr gap analysis for medical device ce marking. This tool will help focusing the requirement introduced by the new mdr. Emergo can. Mdd Mdr Gap Analysis.
From advisera.com
EU MDR vs. MDD Key differences [Infographic] Mdd Mdr Gap Analysis The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to be prepared in order to transition to the mdr. You can download it free, fill it out and if you want send it back to us,. Mdr gap. Mdd Mdr Gap Analysis.
From intellisoft.io
EU Regulation Transitioning from the MDD to MDR Mdd Mdr Gap Analysis Truly the best resource is bsi transition to mdr page. An mdr gap analysis is the process of systematically examining a medical device’s clinical evidence portfolio to determine whether it demonstrates conformity with. You can download it free, fill it out and if you want send it back to us,. Eu mdd to mdr gap analysis for medical device ce. Mdd Mdr Gap Analysis.
From 3r-lifescience.com
Referenz MDDMDR 3R LifeScience Beratung für MedizintechnikUnternehmen Mdd Mdr Gap Analysis The eu medical device regulation 2017/745 (eu mdr) replaces the medical. Specifically, i recommend the following: Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to be prepared in order to transition to the mdr. Ce mdr gap analysis with emergo by ul. Mdr gap assessment tool with comparison. Mdd Mdr Gap Analysis.
From www.greenlight.guru
EU MDR Gap Analysis Tool Free Download Mdd Mdr Gap Analysis Eu mdd to mdr gap analysis for medical device ce marking. This tool will help focusing the requirement introduced by the new mdr. Ce mdr gap analysis with emergo by ul. Mdr gap assessment tool with comparison table of standard requirements for medical devices to assess correlations, identify gaps and streamline. Manufacturers with devices on the market under the mdd. Mdd Mdr Gap Analysis.
From tsquality.ch
MDR vs MDD QUICK COMPARISON TSQuality.ch Mdd Mdr Gap Analysis Manufacturers with devices on the market under the mdd should perform a gap analysis to determine which technical documentation needs to be prepared in order to transition to the mdr. Truly the best resource is bsi transition to mdr page. Mdr gap assessment tool with comparison table of standard requirements for medical devices to assess correlations, identify gaps and streamline.. Mdd Mdr Gap Analysis.