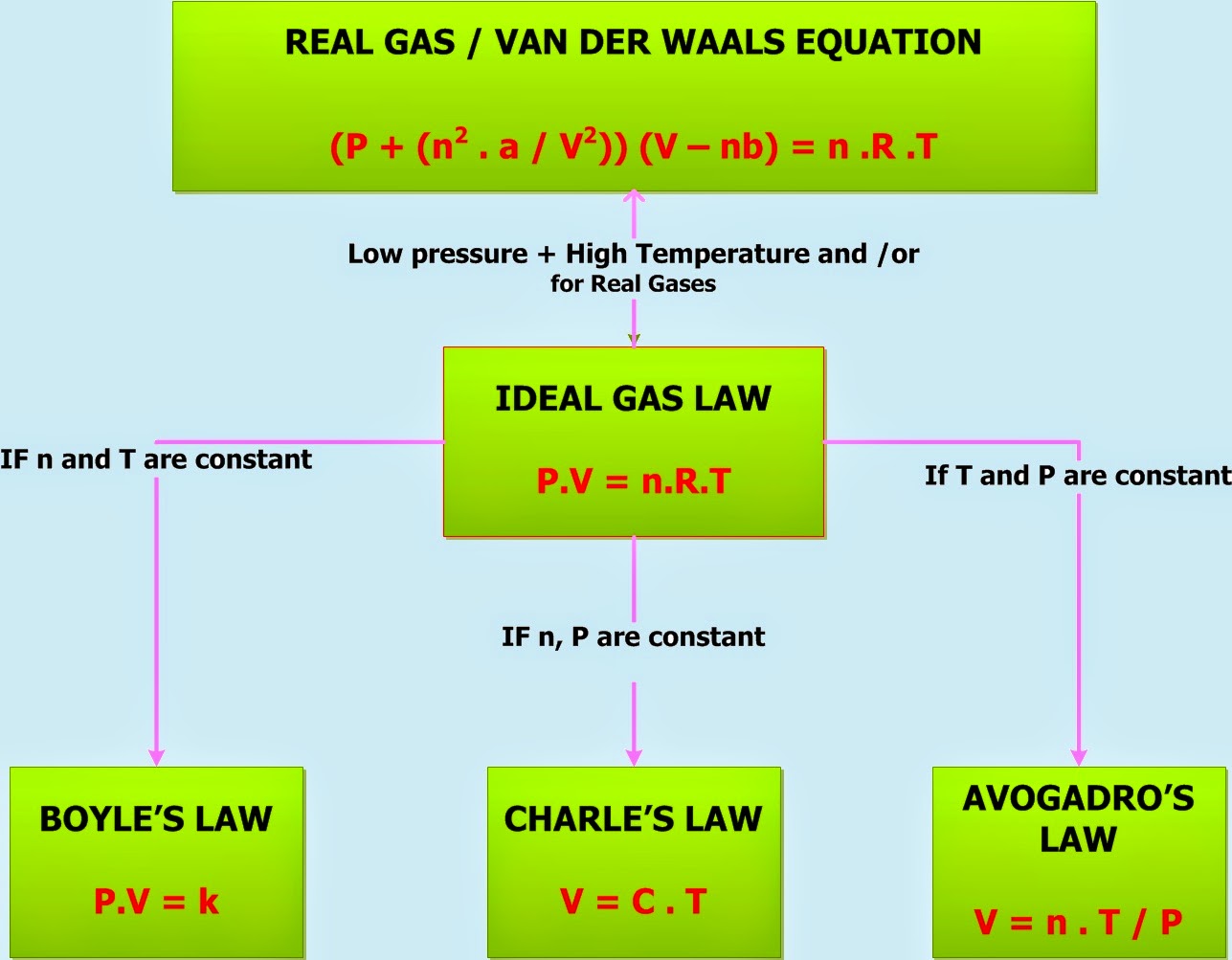
Ideal Gas Properties Temperature And Pressure . Pressure (p), volume (v), number of mole of gas (n), and temperature (t). measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states that. The ideal gas law can be used in stoichiometry. imagine filling a rigid container attached to a pressure gauge with gas and then sealing the container so that no gas may escape. Examine kinetic energy and speed histograms for light and. the ideal gas law relates the four independent physical properties of a gas at any time. Lastly, the constant in the. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. the four gas variables are:
from chem-net.blogspot.com
measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). imagine filling a rigid container attached to a pressure gauge with gas and then sealing the container so that no gas may escape. The ideal gas law can be used in stoichiometry. under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. Examine kinetic energy and speed histograms for light and. the four gas variables are: the ideal gas law relates the four independent physical properties of a gas at any time. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Lastly, the constant in the.
Gas Laws Ideal Gas Law Chemistry Net
Ideal Gas Properties Temperature And Pressure measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. The ideal gas law can be used in stoichiometry. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states that. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the four gas variables are: Examine kinetic energy and speed histograms for light and. imagine filling a rigid container attached to a pressure gauge with gas and then sealing the container so that no gas may escape. Lastly, the constant in the. the ideal gas law relates the four independent physical properties of a gas at any time.
From www.doubtnut.com
Figure shows graphs of pressure vs density for an ideal gas at two Ideal Gas Properties Temperature And Pressure Examine kinetic energy and speed histograms for light and. imagine filling a rigid container attached to a pressure gauge with gas and then sealing the container so that no gas may escape. under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. ideal gas, a gas that. Ideal Gas Properties Temperature And Pressure.
From www.visionlearning.com
Properties of Gases Chemistry Visionlearning Ideal Gas Properties Temperature And Pressure ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states that. under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. Examine kinetic energy and speed histograms for light and. The ideal. Ideal Gas Properties Temperature And Pressure.
From studylib.net
Ideal Gas properties Ideal Gas Properties Temperature And Pressure Examine kinetic energy and speed histograms for light and. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. The ideal gas law can be used in stoichiometry. . Ideal Gas Properties Temperature And Pressure.
From www.slideshare.net
10.1,10.2 Properties Of Gases And Pressure Ideal Gas Properties Temperature And Pressure measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the ideal gas law relates the four independent physical properties of a gas at any time. the four gas variables are: ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure,. Ideal Gas Properties Temperature And Pressure.
From www.slideserve.com
PPT Properties of Gases Chapter 1 PowerPoint Presentation, free Ideal Gas Properties Temperature And Pressure imagine filling a rigid container attached to a pressure gauge with gas and then sealing the container so that no gas may escape. ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states that. Examine kinetic energy and speed histograms for light. Ideal Gas Properties Temperature And Pressure.
From www.slideserve.com
PPT Energy, Enthalpy, and Thermochemistry PowerPoint Presentation Ideal Gas Properties Temperature And Pressure Lastly, the constant in the. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. The ideal gas law can be used in stoichiometry. imagine filling a rigid container attached to a pressure gauge with. Ideal Gas Properties Temperature And Pressure.
From www.slideserve.com
PPT Thermal Physics (Thermodynamics) PowerPoint Presentation ID421070 Ideal Gas Properties Temperature And Pressure measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the four gas variables are: under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. imagine filling a rigid container attached to a pressure gauge with gas. Ideal Gas Properties Temperature And Pressure.
From saylordotorg.github.io
The Behavior of Real Gases Ideal Gas Properties Temperature And Pressure The ideal gas law can be used in stoichiometry. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the ideal gas law relates the four independent physical properties of a gas at any time. under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules.. Ideal Gas Properties Temperature And Pressure.
From www.numerade.com
Student Directions for Gas Properties Chemistry Gas Laws temperature Ideal Gas Properties Temperature And Pressure the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Lastly, the constant in the. The ideal gas law can be used in stoichiometry. under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. imagine filling a. Ideal Gas Properties Temperature And Pressure.
From www.numerade.com
SOLVED It is important to understand how the expressions you derived Ideal Gas Properties Temperature And Pressure Lastly, the constant in the. under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. imagine filling a rigid container attached to a pressure gauge with gas and then sealing the container so that no gas may escape. the ideal gas law is derived from empirical relationships. Ideal Gas Properties Temperature And Pressure.
From www.sciencelearn.org.nz
Properties of gases explained — Science Learning Hub Ideal Gas Properties Temperature And Pressure the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Examine kinetic energy and speed histograms for light and. under various conditions of temperature and pressure, many real. Ideal Gas Properties Temperature And Pressure.
From www.numerade.com
SOLVED One mole of an ideal gas initially at a temperature of Ti = 2 Ideal Gas Properties Temperature And Pressure ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states that. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. measure the temperature and pressure, and discover how the properties. Ideal Gas Properties Temperature And Pressure.
From 2012books.lardbucket.org
The Behavior of Real Gases Ideal Gas Properties Temperature And Pressure Lastly, the constant in the. The ideal gas law can be used in stoichiometry. the four gas variables are: Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the ideal gas law relates the four independent physical properties of a gas at any time. imagine filling a rigid container attached to a pressure. Ideal Gas Properties Temperature And Pressure.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an Ideal Gas Properties Temperature And Pressure under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. Lastly, the constant in the. the four gas variables are: measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the ideal gas law relates the four. Ideal Gas Properties Temperature And Pressure.
From www.grc.nasa.gov
Isentropic Flow Equations Ideal Gas Properties Temperature And Pressure Pressure (p), volume (v), number of mole of gas (n), and temperature (t). under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. Examine kinetic energy and speed histograms for light and. the ideal gas law relates the four independent physical properties of a gas at any time.. Ideal Gas Properties Temperature And Pressure.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Ideal Gas Properties Temperature And Pressure measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the four gas variables are: under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. Lastly, the constant in the. Pressure (p), volume (v), number of mole of. Ideal Gas Properties Temperature And Pressure.
From www.britannica.com
perfect gas law chemistry and physics Britannica Ideal Gas Properties Temperature And Pressure The ideal gas law can be used in stoichiometry. the ideal gas law relates the four independent physical properties of a gas at any time. Lastly, the constant in the. Examine kinetic energy and speed histograms for light and. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the four gas variables are: . Ideal Gas Properties Temperature And Pressure.
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal Ideal Gas Properties Temperature And Pressure imagine filling a rigid container attached to a pressure gauge with gas and then sealing the container so that no gas may escape. the four gas variables are: under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. measure the temperature and pressure, and discover how. Ideal Gas Properties Temperature And Pressure.
From demonstrations.wolfram.com
Temperature Changes in an Ideal Gas Wolfram Demonstrations Project Ideal Gas Properties Temperature And Pressure The ideal gas law can be used in stoichiometry. under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. imagine filling a rigid container attached to a. Ideal Gas Properties Temperature And Pressure.
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal Ideal Gas Properties Temperature And Pressure the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. the four gas variables are: Lastly, the constant in the. Examine kinetic energy and speed histograms for light and. The ideal gas law can be used in stoichiometry. under various conditions of temperature and pressure, many real. Ideal Gas Properties Temperature And Pressure.
From byjus.com
7. The raise in the temperature of a given mass of an ideal gas at Ideal Gas Properties Temperature And Pressure Examine kinetic energy and speed histograms for light and. the ideal gas law relates the four independent physical properties of a gas at any time. ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states that. the four gas variables are:. Ideal Gas Properties Temperature And Pressure.
From www.chegg.com
Solved Thermodynamics 936 TABLE A17 Idealgas properties of Ideal Gas Properties Temperature And Pressure The ideal gas law can be used in stoichiometry. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). Lastly, the constant in the. the four gas variables are: the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. under various conditions of temperature. Ideal Gas Properties Temperature And Pressure.
From www.pinterest.com
Real Gas vs Ideal Gas What Is Neon, It Support Specialist, Ideal Gas Ideal Gas Properties Temperature And Pressure Examine kinetic energy and speed histograms for light and. imagine filling a rigid container attached to a pressure gauge with gas and then sealing the container so that no gas may escape. Lastly, the constant in the. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the. Ideal Gas Properties Temperature And Pressure.
From www.atmo.arizona.edu
Lecture 6 Ideal gas law, rising and sinking air Ideal Gas Properties Temperature And Pressure measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the ideal gas law relates the four independent physical properties of a gas at any time. under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. ideal. Ideal Gas Properties Temperature And Pressure.
From www.youtube.com
11.8 The Ideal Gas Law Pressure, Volume, Temperature, & Moles YouTube Ideal Gas Properties Temperature And Pressure Pressure (p), volume (v), number of mole of gas (n), and temperature (t). The ideal gas law can be used in stoichiometry. ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states that. measure the temperature and pressure, and discover how the. Ideal Gas Properties Temperature And Pressure.
From www.scribd.com
Ideal Gas Properties Table Mod Ideal Gas Properties Temperature And Pressure the four gas variables are: under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Lastly, the constant in the. Examine kinetic energy and speed histograms for. Ideal Gas Properties Temperature And Pressure.
From mmerevise.co.uk
The Ideal Gas Equation MME Ideal Gas Properties Temperature And Pressure under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. Examine kinetic energy and speed histograms for light and. Lastly, the constant in the. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. the four gas. Ideal Gas Properties Temperature And Pressure.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Ideal Gas Properties Temperature And Pressure measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Examine kinetic energy and speed histograms for light and. The ideal gas law can be used in stoichiometry. . Ideal Gas Properties Temperature And Pressure.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Temperature Of Nitrogen Gas Ideal Gas Properties Temperature And Pressure the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the ideal gas law relates. Ideal Gas Properties Temperature And Pressure.
From www.youtube.com
Thermodynamics Fundamentals Thermodynamic Properties Part 5 Ideal Ideal Gas Properties Temperature And Pressure Lastly, the constant in the. under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. the four gas variables are: Pressure (p), volume (v), number of mole of gas (n), and temperature (t). imagine filling a rigid container attached to a pressure gauge with gas and then. Ideal Gas Properties Temperature And Pressure.
From www.grc.nasa.gov
Equation of State Ideal Gas Properties Temperature And Pressure the ideal gas law relates the four independent physical properties of a gas at any time. The ideal gas law can be used in stoichiometry. under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. Lastly, the constant in the. the ideal gas law is derived from. Ideal Gas Properties Temperature And Pressure.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Ideal Gas Properties Temperature And Pressure Examine kinetic energy and speed histograms for light and. Lastly, the constant in the. ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states that. measure the temperature and pressure, and discover how the properties of the gas vary in relation to. Ideal Gas Properties Temperature And Pressure.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Ideal Gas Properties Temperature And Pressure Lastly, the constant in the. imagine filling a rigid container attached to a pressure gauge with gas and then sealing the container so that no gas may escape. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). the four gas variables are: the ideal gas law relates the four independent physical properties of. Ideal Gas Properties Temperature And Pressure.
From www.youtube.com
Ideal Gas Pressure and Volume Dependence on Temperature YouTube Ideal Gas Properties Temperature And Pressure Lastly, the constant in the. under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. imagine filling a rigid container attached to a pressure gauge with gas and. Ideal Gas Properties Temperature And Pressure.
From chem.libretexts.org
Chapter 10.3 The Ideal Gas Law Chemistry LibreTexts Ideal Gas Properties Temperature And Pressure the four gas variables are: under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules. The ideal gas law can be used in stoichiometry. Pressure (p), volume (v), number of mole of gas (n), and temperature (t). measure the temperature and pressure, and discover how the properties. Ideal Gas Properties Temperature And Pressure.