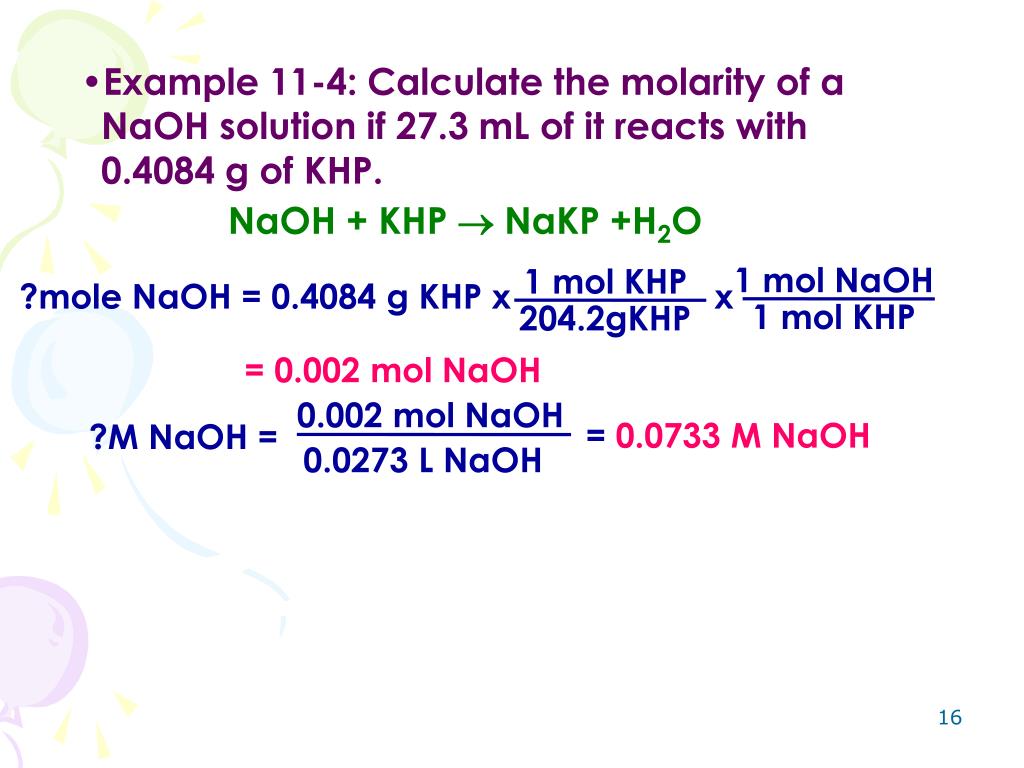
Titration Balanced Equation Formula . For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. the formula for molarity in titration is: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Calculate the ph at these volumes of added base solution: Learn the titration graph and titration equation. \ce{naoh}\) is required to reach the end. What are titrant and analyte. in a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. Molarity = moles of solute/volume of solution (in liters). What is the equivalence point of a titration. The endpoint in a titration is. suppose that a titration is performed and \(20.70 \: a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). →what is the endpoint in a titration?
from www.slideserve.com
the formula for molarity in titration is: The endpoint in a titration is. \ce{naoh}\) is required to reach the end. What are titrant and analyte. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). →what is the endpoint in a titration? in a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. suppose that a titration is performed and \(20.70 \: What is the equivalence point of a titration.
PPT Reactions in Aqueous Solutions II Calculations PowerPoint
Titration Balanced Equation Formula a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). in a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. Calculate the ph at these volumes of added base solution: What are titrant and analyte. The endpoint in a titration is. Molarity = moles of solute/volume of solution (in liters). What is the equivalence point of a titration. suppose that a titration is performed and \(20.70 \: For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. the formula for molarity in titration is: Learn the titration graph and titration equation. →what is the endpoint in a titration? \ce{naoh}\) is required to reach the end. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]).
From dxopqqjgm.blob.core.windows.net
Titration Concentration Formula at Stephen Hansen blog Titration Balanced Equation Formula Learn the titration graph and titration equation. the formula for molarity in titration is: Calculate the ph at these volumes of added base solution: in a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. a titration is a volumetric. Titration Balanced Equation Formula.
From www.slideserve.com
PPT Reactions in Aqueous Solutions II Calculations PowerPoint Titration Balanced Equation Formula The endpoint in a titration is. \ce{naoh}\) is required to reach the end. Molarity = moles of solute/volume of solution (in liters). a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. →what is the endpoint in a titration? For a. Titration Balanced Equation Formula.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Titration Balanced Equation Formula Learn the titration graph and titration equation. \ce{naoh}\) is required to reach the end. →what is the endpoint in a titration? a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). the formula for molarity in titration is: What. Titration Balanced Equation Formula.
From www.numerade.com
SOLVED The balanced equation for the titration of oxalic acid with Titration Balanced Equation Formula a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). →what is the endpoint in a titration? What is the equivalence point of a titration. The endpoint in a titration is. What are titrant and analyte. Molarity = moles of. Titration Balanced Equation Formula.
From www.numerade.com
SOLVED Write a balanced equation for the corresponding titration Titration Balanced Equation Formula What is the equivalence point of a titration. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. \ce{naoh}\) is required to reach the end. Molarity = moles of solute/volume of solution (in liters). a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Titration Balanced Equation Formula.
From www.chegg.com
Solved The balanced equation for the titration reaction is Titration Balanced Equation Formula the formula for molarity in titration is: Learn the titration graph and titration equation. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at. Titration Balanced Equation Formula.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration Balanced Equation Formula The endpoint in a titration is. What is the equivalence point of a titration. What are titrant and analyte. Learn the titration graph and titration equation. Molarity = moles of solute/volume of solution (in liters). the formula for molarity in titration is: suppose that a titration is performed and \(20.70 \: a titration is a volumetric technique. Titration Balanced Equation Formula.
From celjgvoh.blob.core.windows.net
Balanced Equation For The Titration at Barbara Hulbert blog Titration Balanced Equation Formula The endpoint in a titration is. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). What are titrant and analyte. suppose that a titration is performed and \(20.70 \: What is the equivalence point of a titration. →what. Titration Balanced Equation Formula.
From www.chegg.com
Solved Titrations Balanced equation is needed for every Titration Balanced Equation Formula The endpoint in a titration is. What is the equivalence point of a titration. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Molarity = moles of solute/volume of solution (in liters). What are titrant and analyte. the formula. Titration Balanced Equation Formula.
From dxompffry.blob.core.windows.net
Titration Formula Class 12 at Donald Rios blog Titration Balanced Equation Formula a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. What is the equivalence point of a titration. The endpoint in a titration is. Learn the titration graph and titration equation. suppose that a titration is performed and \(20.70 \:. Titration Balanced Equation Formula.
From celjgvoh.blob.core.windows.net
Balanced Equation For The Titration at Barbara Hulbert blog Titration Balanced Equation Formula Learn the titration graph and titration equation. the formula for molarity in titration is: What is the equivalence point of a titration. The endpoint in a titration is. What are titrant and analyte. →what is the endpoint in a titration? in a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to. Titration Balanced Equation Formula.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Balanced Equation Formula a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. What is the equivalence point of a titration. Molarity = moles of solute/volume of solution (in liters). the formula for molarity in titration is: The endpoint in a titration is.. Titration Balanced Equation Formula.
From shiken.ai
Titrations Titration Balanced Equation Formula Learn the titration graph and titration equation. What are titrant and analyte. →what is the endpoint in a titration? \ce{naoh}\) is required to reach the end. in a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. suppose that a titration. Titration Balanced Equation Formula.
From www.youtube.com
13 Titration Sample Calculation YouTube Titration Balanced Equation Formula the formula for molarity in titration is: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. suppose that a titration is performed and \(20.70 \: a titration is carried out for 25.00 ml of 0.100 m hcl. Titration Balanced Equation Formula.
From www.numerade.com
SOLVED The balanced equation for the titration of oxalic acid with Titration Balanced Equation Formula a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). What are titrant and analyte. What is the equivalence point of a titration. The endpoint in a titration is. the formula for molarity in titration is: For a neutralization. Titration Balanced Equation Formula.
From www.vrogue.co
Solved The Balanced Equation For The Titration Of Oxa vrogue.co Titration Balanced Equation Formula in a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. The endpoint in a titration is. Calculate the ph at these volumes of added base solution: Molarity = moles of solute/volume of solution (in liters). a titration is a volumetric. Titration Balanced Equation Formula.
From www.youtube.com
Titration Stoichiometry HCl and Ba(OH)2 YouTube Titration Balanced Equation Formula What are titrant and analyte. in a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Titration Balanced Equation Formula.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Titration Balanced Equation Formula Learn the titration graph and titration equation. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Molarity = moles of solute/volume of solution (in liters). \ce{naoh}\) is required to reach the end. Calculate the ph at these volumes of. Titration Balanced Equation Formula.
From www.youtube.com
Iodine and sodium thiosulfate redox titration calculations ALevel Titration Balanced Equation Formula Molarity = moles of solute/volume of solution (in liters). a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution. Titration Balanced Equation Formula.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Balanced Equation Formula Learn the titration graph and titration equation. What are titrant and analyte. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Molarity = moles of solute/volume of solution (in liters). \ce{naoh}\) is required to reach the end. the. Titration Balanced Equation Formula.
From www.chegg.com
Solved Balanced Chemical Equation for Titration Reaction Titration Balanced Equation Formula Learn the titration graph and titration equation. The endpoint in a titration is. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Molarity = moles of solute/volume of solution (in liters). suppose that a titration is performed and \(20.70. Titration Balanced Equation Formula.
From www.youtube.com
Ammonium Iron (II) Sulphate vs KMnO4 Titration Calculations Example Titration Balanced Equation Formula Learn the titration graph and titration equation. the formula for molarity in titration is: Calculate the ph at these volumes of added base solution: The endpoint in a titration is. What are titrant and analyte. in a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh −. Titration Balanced Equation Formula.
From www.reddit.com
Don't I need to know the other chemical formula to write the balanced Titration Balanced Equation Formula Calculate the ph at these volumes of added base solution: The endpoint in a titration is. What are titrant and analyte. suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of. Titration Balanced Equation Formula.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Balanced Equation Formula The endpoint in a titration is. What is the equivalence point of a titration. →what is the endpoint in a titration? For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Molarity = moles of solute/volume of solution (in liters). a titration is a volumetric technique in which a solution of one. Titration Balanced Equation Formula.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube Titration Balanced Equation Formula The endpoint in a titration is. the formula for molarity in titration is: Molarity = moles of solute/volume of solution (in liters). What are titrant and analyte. →what is the endpoint in a titration? Learn the titration graph and titration equation. Calculate the ph at these volumes of added base solution: a titration is carried out for 25.00. Titration Balanced Equation Formula.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration Balanced Equation Formula Calculate the ph at these volumes of added base solution: The endpoint in a titration is. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Learn the titration graph and titration equation. in a balanced neutralization equation, the moles. Titration Balanced Equation Formula.
From www.slideserve.com
PPT Titrations PowerPoint Presentation ID5572517 Titration Balanced Equation Formula What is the equivalence point of a titration. the formula for molarity in titration is: Molarity = moles of solute/volume of solution (in liters). →what is the endpoint in a titration? The endpoint in a titration is. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of. Titration Balanced Equation Formula.
From www.vrogue.co
Solved The Balanced Equation For The Titration Of Oxa vrogue.co Titration Balanced Equation Formula the formula for molarity in titration is: Learn the titration graph and titration equation. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown. Titration Balanced Equation Formula.
From exonhrnmo.blob.core.windows.net
Titration Formula Ratio at Sarah Campbell blog Titration Balanced Equation Formula What are titrant and analyte. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. the formula for molarity in titration is: . Titration Balanced Equation Formula.
From www.chegg.com
Balanced equation for the titration reaction in the Titration Balanced Equation Formula in a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Calculate the. Titration Balanced Equation Formula.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Balanced Equation Formula For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. What are titrant and analyte. Learn the titration graph and titration equation. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. →what is. Titration Balanced Equation Formula.
From dxopqqjgm.blob.core.windows.net
Titration Concentration Formula at Stephen Hansen blog Titration Balanced Equation Formula \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: Calculate the ph at these volumes of added base solution: in a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. a titration is. Titration Balanced Equation Formula.
From celjgvoh.blob.core.windows.net
Balanced Equation For The Titration at Barbara Hulbert blog Titration Balanced Equation Formula What is the equivalence point of a titration. the formula for molarity in titration is: The endpoint in a titration is. What are titrant and analyte. Learn the titration graph and titration equation. suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. →what is the endpoint in a titration? a. Titration Balanced Equation Formula.
From www.youtube.com
Basic Titrations Chemistry Tutorial YouTube Titration Balanced Equation Formula For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Calculate the ph at these volumes of added base solution: What is the equivalence point of a titration. →what is the endpoint in a titration? a titration is a volumetric technique in which a solution of one reactant (the titrant) is added. Titration Balanced Equation Formula.
From morioh.com
Acid Base Titration Curves PH Calculations Titration Balanced Equation Formula Learn the titration graph and titration equation. What are titrant and analyte. Calculate the ph at these volumes of added base solution: \ce{naoh}\) is required to reach the end. →what is the endpoint in a titration? What is the equivalence point of a titration. suppose that a titration is performed and \(20.70 \: For a neutralization reaction with a. Titration Balanced Equation Formula.