Dilution Examples Chemistry . Calculating concentration of a solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Find the dilution formula and examples of stock. Learn how to change concentrations of solutions by dilution or concentration. Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Concentration can be calculated using the following equation: Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration: See two examples of dilution problems. Changes in concentrations (specifically a reduction) are called dilutions.
from spmchemistry.blog.onlinetuition.com.my
Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. See two examples of dilution problems. Find the dilution formula and examples of stock. Concentration can be calculated using the following equation: Changes in concentrations (specifically a reduction) are called dilutions. Calculating concentration of a solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration: Learn how to change concentrations of solutions by dilution or concentration. Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant.
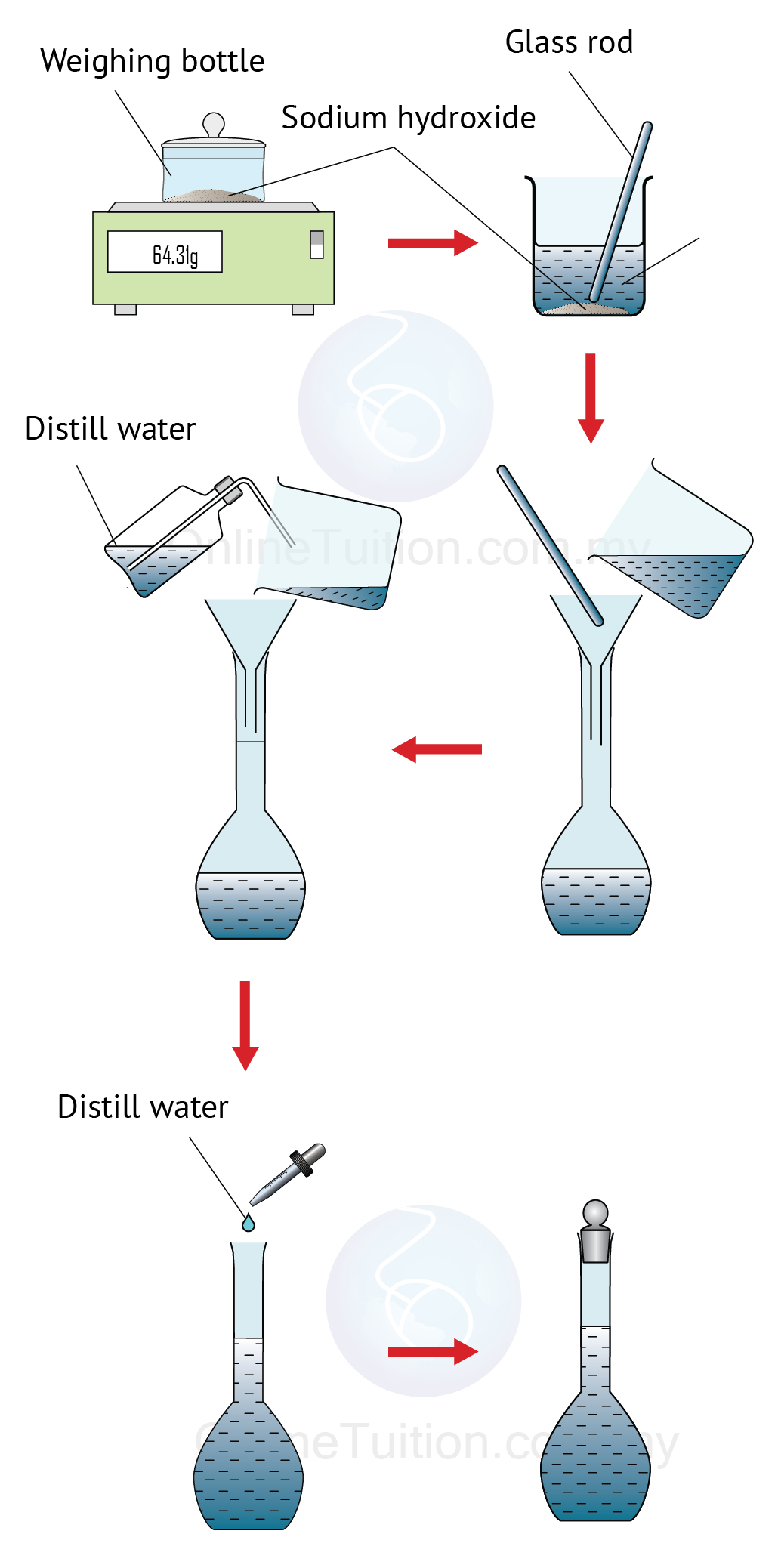
Dilution SPM Chemistry
Dilution Examples Chemistry Dilution is a process used to lower the concentration of the original solution by adding more solvent. Concentration can be calculated using the following equation: Learn how to change concentrations of solutions by dilution or concentration. See two examples of dilution problems. Changes in concentrations (specifically a reduction) are called dilutions. Calculating concentration of a solution. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Find the dilution formula and examples of stock. \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration:
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilution Examples Chemistry \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration: Calculating concentration of a solution. Find the dilution formula and examples of stock. Learn how to change concentrations of solutions by dilution or concentration. Dilution is a process used to lower the concentration of the original solution by adding more solvent. See two examples of dilution problems. Changes in concentrations (specifically. Dilution Examples Chemistry.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Examples Chemistry Calculating concentration of a solution. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Changes in concentrations (specifically a reduction) are called dilutions. Find the dilution formula and examples of stock. Learn how to change concentrations of solutions by dilution or concentration. Dilution is a process used to lower the. Dilution Examples Chemistry.
From spmchemistry.blog.onlinetuition.com.my
Dilution SPM Chemistry Dilution Examples Chemistry Find the dilution formula and examples of stock. Calculating concentration of a solution. Learn how to change concentrations of solutions by dilution or concentration. See two examples of dilution problems. Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Learn how to calculate the concentration and volume of a diluted solution. Dilution Examples Chemistry.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration Dilution Examples Chemistry \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration: Calculating concentration of a solution. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to dilute a solution by adding solvent and. Dilution Examples Chemistry.
From www.studocu.com
General Chemistry 9 Dilution, and some examples Concentrations Of Dilution Examples Chemistry Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. See two examples of dilution problems. Concentration can be calculated using the following equation: Calculating concentration of a solution. Changes in concentrations (specifically. Dilution Examples Chemistry.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Dilution Examples Chemistry See two examples of dilution problems. Find the dilution formula and examples of stock. Calculating concentration of a solution. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Concentration can be calculated using. Dilution Examples Chemistry.
From what-is-this.net
dilution definition What is Dilution Examples Chemistry \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration: Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Concentration can be calculated using the following equation: See two examples of dilution problems. Calculating concentration of a solution. Changes in concentrations (specifically a reduction) are called dilutions. Learn how to change. Dilution Examples Chemistry.
From facts.net
13 Surprising Facts About Dilution Dilution Examples Chemistry \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration: Changes in concentrations (specifically a reduction) are called dilutions. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. See two examples of dilution problems. Learn how to change concentrations of solutions by dilution or concentration. Find the dilution formula and examples. Dilution Examples Chemistry.
From www.youtube.com
Beginning Chemistry Solution Dilution YouTube Dilution Examples Chemistry Concentration can be calculated using the following equation: Changes in concentrations (specifically a reduction) are called dilutions. Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Find the dilution formula and examples of stock. Dilution is a process used to lower the concentration of the original solution by adding more solvent.. Dilution Examples Chemistry.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Examples Chemistry Calculating concentration of a solution. Concentration can be calculated using the following equation: Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Changes in concentrations (specifically a reduction) are called dilutions. See two examples. Dilution Examples Chemistry.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Examples Chemistry Dilution is a process used to lower the concentration of the original solution by adding more solvent. See two examples of dilution problems. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Concentration can be calculated using the following equation: Learn how to dilute a solution by adding solvent and. Dilution Examples Chemistry.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution Examples Chemistry Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to change concentrations of solutions by dilution or concentration. Find the dilution formula and examples of stock. Changes in concentrations (specifically a reduction). Dilution Examples Chemistry.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Dilution Examples Chemistry Calculating concentration of a solution. Changes in concentrations (specifically a reduction) are called dilutions. Learn how to change concentrations of solutions by dilution or concentration. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Dilution is a process used to lower the concentration of the original solution by adding more. Dilution Examples Chemistry.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilution Examples Chemistry Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Concentration can be calculated using the following equation: Learn how to change concentrations of solutions by dilution or concentration. \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration: Changes in concentrations (specifically a reduction) are called dilutions. Learn how to calculate the. Dilution Examples Chemistry.
From stock.adobe.com
Scientific experiment diagram show concept of serial dilution for Dilution Examples Chemistry Calculating concentration of a solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. See two examples of dilution problems. Learn how to change concentrations of solutions by dilution or concentration. Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Concentration can be. Dilution Examples Chemistry.
From www.youtube.com
What is Dilute Solution? Chemistry YouTube Dilution Examples Chemistry Calculating concentration of a solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. See two examples of dilution problems. Changes in concentrations (specifically a reduction) are called dilutions. Learn how to change concentrations of solutions by dilution or concentration. Learn how to calculate the concentration and volume of a diluted solution. Dilution Examples Chemistry.
From www.slideserve.com
PPT Serial Dilution PowerPoint Presentation, free download ID3119682 Dilution Examples Chemistry Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Calculating concentration of a solution. Concentration can be calculated using the following equation: Find the dilution formula and examples of stock. Learn how to change. Dilution Examples Chemistry.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Examples Chemistry Calculating concentration of a solution. Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. See two examples of dilution problems. Changes in concentrations (specifically a reduction) are called dilutions. \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration: Learn how to change concentrations of solutions by dilution or concentration. Dilution is. Dilution Examples Chemistry.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Examples Chemistry Changes in concentrations (specifically a reduction) are called dilutions. See two examples of dilution problems. Learn how to change concentrations of solutions by dilution or concentration. Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Concentration can be calculated using the following equation: \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}}. Dilution Examples Chemistry.
From www.youtube.com
Testing the validity of Ostwald’s dilution law/Determination of Dilution Examples Chemistry Changes in concentrations (specifically a reduction) are called dilutions. Concentration can be calculated using the following equation: Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Calculating concentration of a solution. Find the. Dilution Examples Chemistry.
From www.youtube.com
Serial Dilution Required Practical Revision for Biology and Chemistry Dilution Examples Chemistry Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Find the dilution formula and examples of stock. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to change concentrations of solutions by dilution or concentration. Learn how to calculate the concentration. Dilution Examples Chemistry.
From yellowdig263.weebly.com
Serial Dilution Vs Parallel Dilution Dilution Examples Chemistry Learn how to change concentrations of solutions by dilution or concentration. Calculating concentration of a solution. Concentration can be calculated using the following equation: \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration: Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to calculate the concentration and volume of a. Dilution Examples Chemistry.
From socratic.org
mL of solution has a concentration of 3.0 M. How much water needs to be Dilution Examples Chemistry \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration: Concentration can be calculated using the following equation: Find the dilution formula and examples of stock. Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and. Dilution Examples Chemistry.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation, free download ID Dilution Examples Chemistry Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Find the dilution formula and examples of stock. See two examples of dilution problems. Learn how to change concentrations of solutions by dilution or. Dilution Examples Chemistry.
From socratic.org
How can I calculate the dilution factor using concentration? Socratic Dilution Examples Chemistry Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Concentration can be calculated using the following equation: Calculating concentration of a solution. Learn how to change concentrations of solutions by dilution or concentration. Dilution is a process used to lower the concentration of the original solution by adding more solvent.. Dilution Examples Chemistry.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5812279 Dilution Examples Chemistry \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration: Learn how to change concentrations of solutions by dilution or concentration. See two examples of dilution problems. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Learn how to dilute a solution by adding solvent and keep the number of moles. Dilution Examples Chemistry.
From www.youtube.com
What Is Dilution? Chemistry Matters YouTube Dilution Examples Chemistry Learn how to change concentrations of solutions by dilution or concentration. Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Changes in concentrations (specifically a reduction) are called dilutions. Concentration can be calculated using the following equation: See two examples of dilution problems. \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}}. Dilution Examples Chemistry.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Examples Chemistry See two examples of dilution problems. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Find the dilution formula and examples of stock. Learn how to change concentrations of solutions by dilution or concentration. Concentration can be calculated using the following equation: Changes in concentrations (specifically a reduction) are called dilutions. \text. Dilution Examples Chemistry.
From www.toppr.com
Dilution Formula Definition, Concepts and Examples Dilution Examples Chemistry Learn how to change concentrations of solutions by dilution or concentration. Find the dilution formula and examples of stock. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Calculating concentration of a. Dilution Examples Chemistry.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of Dilution Examples Chemistry Concentration can be calculated using the following equation: Calculating concentration of a solution. \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration: Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Find the. Dilution Examples Chemistry.
From www.thoughtco.com
Dilution Calculations From Stock Solutions in Chemistry Dilution Examples Chemistry See two examples of dilution problems. Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Concentration can be calculated using the following equation: Learn how to change concentrations of solutions by dilution or concentration. Changes in concentrations (specifically a reduction) are called dilutions. Calculating concentration of a solution. \text {concentration}=\dfrac {\text. Dilution Examples Chemistry.
From www.pinterest.com
Dilute Flashcards, Easy science, Science student Dilution Examples Chemistry Changes in concentrations (specifically a reduction) are called dilutions. Dilution is a process used to lower the concentration of the original solution by adding more solvent. \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration: Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Learn how to calculate the concentration and. Dilution Examples Chemistry.
From www.chegg.com
Solved Serial dilution is a common technique used in Dilution Examples Chemistry \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration: See two examples of dilution problems. Changes in concentrations (specifically a reduction) are called dilutions. Concentration can be calculated using the following equation: Calculating concentration of a solution. Find the dilution formula and examples of stock. Learn how to dilute a solution by adding solvent and keep the number of moles. Dilution Examples Chemistry.
From ar.inspiredpencil.com
Real Life Examples Of Density Dilution Examples Chemistry Changes in concentrations (specifically a reduction) are called dilutions. Learn how to dilute a solution by adding solvent and keep the number of moles of solute constant. Find the dilution formula and examples of stock. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Calculating concentration of a solution. \text. Dilution Examples Chemistry.
From kayliefernandez.blogspot.com
PreAP Chemistry Dilutions Dilution Examples Chemistry \text {concentration}=\dfrac {\text {number of moles}} {\text {volume}} concentration: Concentration can be calculated using the following equation: Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Learn how to change concentrations of solutions by dilution or concentration. Dilution is a process used to lower the concentration of the original solution. Dilution Examples Chemistry.