Galvanic Corrosion Cathode Anode Ratio . It is found that the galvanic corrosion effect on carbon steel anode increases with the cathode/anode area ratios, and decreases with the increasing concentration of s2− in the solution. The larger the cathode compared with the. The relative surface area of the anode to the cathode impacts galvanic corrosion rates. Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. A layer of sulfide film is formed on carbon As a given amount of current flows in a galvanic couple,. Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. The surface area effect tells us that if the area of the anode is much smaller than that of the cathode, then corrosion of the anode will be much
from www.numerade.com
Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. A layer of sulfide film is formed on carbon Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. The surface area effect tells us that if the area of the anode is much smaller than that of the cathode, then corrosion of the anode will be much The relative surface area of the anode to the cathode impacts galvanic corrosion rates. As a given amount of current flows in a galvanic couple,. It is found that the galvanic corrosion effect on carbon steel anode increases with the cathode/anode area ratios, and decreases with the increasing concentration of s2− in the solution. The larger the cathode compared with the. The area ratio of the anode to cathode plays a dominant role in galvanic corrosion.
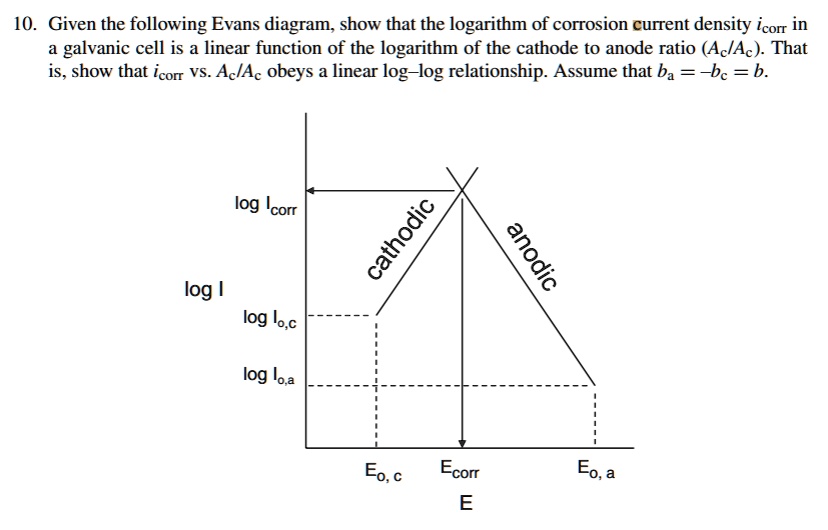
SOLVED Given the following Evans diagram, show that the logarithm of
Galvanic Corrosion Cathode Anode Ratio Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. The larger the cathode compared with the. The surface area effect tells us that if the area of the anode is much smaller than that of the cathode, then corrosion of the anode will be much A layer of sulfide film is formed on carbon The relative surface area of the anode to the cathode impacts galvanic corrosion rates. Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. As a given amount of current flows in a galvanic couple,. It is found that the galvanic corrosion effect on carbon steel anode increases with the cathode/anode area ratios, and decreases with the increasing concentration of s2− in the solution.
From mungfali.com
Galvanic Corrosion Chart Metals Galvanic Corrosion Cathode Anode Ratio A layer of sulfide film is formed on carbon The relative surface area of the anode to the cathode impacts galvanic corrosion rates. As a given amount of current flows in a galvanic couple,. The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. The surface area effect tells us that if the area of. Galvanic Corrosion Cathode Anode Ratio.
From slideplayer.com
FORMS OF CORROSION CHAPTER 3 Chapter Outlines ppt download Galvanic Corrosion Cathode Anode Ratio The surface area effect tells us that if the area of the anode is much smaller than that of the cathode, then corrosion of the anode will be much The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. The larger the cathode compared with the. As a given amount of current flows in a. Galvanic Corrosion Cathode Anode Ratio.
From www.corrosionpedia.com
Galvanic Cathodic Protection Galvanic Corrosion Cathode Anode Ratio Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. The relative surface area of the anode to the cathode impacts galvanic corrosion rates. Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. It is found that the galvanic corrosion effect on. Galvanic Corrosion Cathode Anode Ratio.
From mavink.com
Galvanic Cell Cathode Anode Galvanic Corrosion Cathode Anode Ratio The larger the cathode compared with the. Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. As a given amount of current flows in a galvanic couple,. Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. The area ratio of the. Galvanic Corrosion Cathode Anode Ratio.
From www.corrosionclinic.com
A TopRated Software Tool For Predicting Galvanic Galvanic Corrosion Cathode Anode Ratio The surface area effect tells us that if the area of the anode is much smaller than that of the cathode, then corrosion of the anode will be much The larger the cathode compared with the. As a given amount of current flows in a galvanic couple,. The relative surface area of the anode to the cathode impacts galvanic corrosion. Galvanic Corrosion Cathode Anode Ratio.
From www.researchgate.net
(PDF) Area Ratio of Cathode/Anode Effect on the Galvanic Corrosion of Galvanic Corrosion Cathode Anode Ratio As a given amount of current flows in a galvanic couple,. The larger the cathode compared with the. The surface area effect tells us that if the area of the anode is much smaller than that of the cathode, then corrosion of the anode will be much The area ratio of the anode to cathode plays a dominant role in. Galvanic Corrosion Cathode Anode Ratio.
From www.slideserve.com
PPT 7. Galvanic Corrosion PowerPoint Presentation, free download ID Galvanic Corrosion Cathode Anode Ratio The surface area effect tells us that if the area of the anode is much smaller than that of the cathode, then corrosion of the anode will be much The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass%. Galvanic Corrosion Cathode Anode Ratio.
From www.slideserve.com
PPT 7. Galvanic Corrosion PowerPoint Presentation, free download ID Galvanic Corrosion Cathode Anode Ratio The larger the cathode compared with the. The surface area effect tells us that if the area of the anode is much smaller than that of the cathode, then corrosion of the anode will be much It is found that the galvanic corrosion effect on carbon steel anode increases with the cathode/anode area ratios, and decreases with the increasing concentration. Galvanic Corrosion Cathode Anode Ratio.
From www.researchgate.net
The corrosion cell is a galvanic cell in which the anode and cathode Galvanic Corrosion Cathode Anode Ratio The surface area effect tells us that if the area of the anode is much smaller than that of the cathode, then corrosion of the anode will be much Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. It is found that the galvanic corrosion effect on carbon steel anode. Galvanic Corrosion Cathode Anode Ratio.
From www.slideserve.com
PPT Modeling Galvanic Corrosion PowerPoint Presentation, free Galvanic Corrosion Cathode Anode Ratio As a given amount of current flows in a galvanic couple,. The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass%. Galvanic Corrosion Cathode Anode Ratio.
From www.studocu.com
Surface Area Effects in a Galvanic Situation The larger the cathode Galvanic Corrosion Cathode Anode Ratio A layer of sulfide film is formed on carbon Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. As a given amount of current flows in a galvanic couple,. The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. Galvanic corrosion at the joint of azx611. Galvanic Corrosion Cathode Anode Ratio.
From ifitsmoving.com
Galvanic Corrosion Some Observations From the Field If It's Moving Galvanic Corrosion Cathode Anode Ratio A layer of sulfide film is formed on carbon Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. The larger the cathode compared with the. As a given amount of current flows. Galvanic Corrosion Cathode Anode Ratio.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Galvanic Corrosion Cathode Anode Ratio The larger the cathode compared with the. As a given amount of current flows in a galvanic couple,. Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. A layer of sulfide film is formed on carbon The relative surface area of the anode to the cathode impacts galvanic corrosion rates.. Galvanic Corrosion Cathode Anode Ratio.
From www.researchgate.net
Steady state galvaniccurrent density between 6061T6 Al and CFR PMC Galvanic Corrosion Cathode Anode Ratio The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. As a given amount of current flows in a galvanic couple,. Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. The larger the cathode compared with the. A layer of sulfide film is formed on carbon. Galvanic Corrosion Cathode Anode Ratio.
From www.thoughtco.com
How to Define Anode and Cathode Galvanic Corrosion Cathode Anode Ratio A layer of sulfide film is formed on carbon It is found that the galvanic corrosion effect on carbon steel anode increases with the cathode/anode area ratios, and decreases with the increasing concentration of s2− in the solution. Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. The surface area. Galvanic Corrosion Cathode Anode Ratio.
From www.slideserve.com
PPT 5 Ways to Avoid Galvanic Corrosion PowerPoint Presentation, free Galvanic Corrosion Cathode Anode Ratio The larger the cathode compared with the. The surface area effect tells us that if the area of the anode is much smaller than that of the cathode, then corrosion of the anode will be much The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. A layer of sulfide film is formed on carbon. Galvanic Corrosion Cathode Anode Ratio.
From www.researchgate.net
The effect of the surface area ratio of anode to cathode and the Galvanic Corrosion Cathode Anode Ratio The surface area effect tells us that if the area of the anode is much smaller than that of the cathode, then corrosion of the anode will be much The relative surface area of the anode to the cathode impacts galvanic corrosion rates. Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic. Galvanic Corrosion Cathode Anode Ratio.
From www.researchgate.net
Predicted corrosion rate vs. anodetocathode ratio for a 4130 steel Galvanic Corrosion Cathode Anode Ratio As a given amount of current flows in a galvanic couple,. Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. The relative surface area of the anode to the cathode impacts galvanic corrosion rates. The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. The. Galvanic Corrosion Cathode Anode Ratio.
From www.slideserve.com
PPT Modeling Galvanic Corrosion PowerPoint Presentation, free Galvanic Corrosion Cathode Anode Ratio The larger the cathode compared with the. The surface area effect tells us that if the area of the anode is much smaller than that of the cathode, then corrosion of the anode will be much The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. A layer of sulfide film is formed on carbon. Galvanic Corrosion Cathode Anode Ratio.
From www.linkedin.com
Anil Vashishta on LinkedIn galvanic corrosion cathode anode Galvanic Corrosion Cathode Anode Ratio The larger the cathode compared with the. Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. It is found that the galvanic corrosion effect on carbon steel anode increases with the cathode/anode area ratios, and decreases with the increasing concentration of s2− in the solution. The area ratio of the. Galvanic Corrosion Cathode Anode Ratio.
From www.slideserve.com
PPT 7. Galvanic Corrosion PowerPoint Presentation, free download ID Galvanic Corrosion Cathode Anode Ratio The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. The relative surface area of the anode to the cathode impacts galvanic corrosion rates. Another important factor in galvanic corrosion is the area effect or the. Galvanic Corrosion Cathode Anode Ratio.
From armoloy.com
Understanding Galvanic Corrosion Concepts, Causes, and Prevention Galvanic Corrosion Cathode Anode Ratio The relative surface area of the anode to the cathode impacts galvanic corrosion rates. Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. A layer of sulfide film is formed on carbon It is found that the galvanic corrosion effect on carbon steel anode increases with the cathode/anode area ratios,. Galvanic Corrosion Cathode Anode Ratio.
From www.semanticscholar.org
Figure 1 from Effect of area ratio on the galvanic corrosion of AZX611 Galvanic Corrosion Cathode Anode Ratio The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. The surface area effect tells us that if the area of the anode is much smaller than that of the cathode, then corrosion of the anode will be much A layer of sulfide film is formed on carbon The larger the cathode compared with the.. Galvanic Corrosion Cathode Anode Ratio.
From www.researchgate.net
Galvanic corrosion rate vs skirt lengthtosalt loading ratio l/m from Galvanic Corrosion Cathode Anode Ratio As a given amount of current flows in a galvanic couple,. The larger the cathode compared with the. A layer of sulfide film is formed on carbon Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. It is found that the galvanic corrosion effect on carbon steel anode increases with. Galvanic Corrosion Cathode Anode Ratio.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Galvanic Corrosion Cathode Anode Ratio It is found that the galvanic corrosion effect on carbon steel anode increases with the cathode/anode area ratios, and decreases with the increasing concentration of s2− in the solution. Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. The relative surface area of the anode to the cathode impacts galvanic corrosion. Galvanic Corrosion Cathode Anode Ratio.
From www.semanticscholar.org
Figure 2 from Effect of area ratio on the galvanic corrosion of AZX611 Galvanic Corrosion Cathode Anode Ratio The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. It is found that the galvanic corrosion effect on carbon steel anode increases with the cathode/anode area ratios, and decreases with the increasing concentration of s2− in the solution. Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass%. Galvanic Corrosion Cathode Anode Ratio.
From www.safefoodfactory.com
Stainless Steel in Contact with Other Metallic Materials Safe Food Galvanic Corrosion Cathode Anode Ratio The relative surface area of the anode to the cathode impacts galvanic corrosion rates. It is found that the galvanic corrosion effect on carbon steel anode increases with the cathode/anode area ratios, and decreases with the increasing concentration of s2− in the solution. As a given amount of current flows in a galvanic couple,. A layer of sulfide film is. Galvanic Corrosion Cathode Anode Ratio.
From www.slideserve.com
PPT 7. Galvanic Corrosion PowerPoint Presentation, free download ID Galvanic Corrosion Cathode Anode Ratio The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. The relative surface area of the anode to the cathode impacts galvanic corrosion rates. Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. The surface area effect tells us that if the area of the anode. Galvanic Corrosion Cathode Anode Ratio.
From www.numerade.com
SOLVED Given the following Evans diagram, show that the logarithm of Galvanic Corrosion Cathode Anode Ratio The larger the cathode compared with the. A layer of sulfide film is formed on carbon Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. The surface area effect tells us that if the area. Galvanic Corrosion Cathode Anode Ratio.
From www.researchgate.net
(PDF) Area Ratio of Cathode/Anode Effect on the Galvanic Corrosion of Galvanic Corrosion Cathode Anode Ratio It is found that the galvanic corrosion effect on carbon steel anode increases with the cathode/anode area ratios, and decreases with the increasing concentration of s2− in the solution. The larger the cathode compared with the. Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. As a given amount of current. Galvanic Corrosion Cathode Anode Ratio.
From chempedia.info
Galvanic corrosion anode/cathode area ratio Big Chemical Encyclopedia Galvanic Corrosion Cathode Anode Ratio Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. The larger the cathode compared with the. It is found that the galvanic corrosion effect on carbon steel anode increases with the cathode/anode area ratios, and decreases with the increasing concentration of s2− in the solution. The surface area effect tells us. Galvanic Corrosion Cathode Anode Ratio.
From www.icorr.org
Galvanic Corrosion the importance of designingout corrosion hotspot Galvanic Corrosion Cathode Anode Ratio Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. Galvanic corrosion at the joint of azx611 magnesium (anode) and a6n01 aluminum (cathode) in 1 mass% nacl solution with. The surface area effect tells us that if the area of the anode is much smaller than that of the cathode, then corrosion. Galvanic Corrosion Cathode Anode Ratio.
From www.solaracks.com
What is galvanic corrosion/bimetallic corrosion/dissimilar metal Galvanic Corrosion Cathode Anode Ratio The surface area effect tells us that if the area of the anode is much smaller than that of the cathode, then corrosion of the anode will be much The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to. Galvanic Corrosion Cathode Anode Ratio.
From saylordotorg.github.io
Corrosion Galvanic Corrosion Cathode Anode Ratio The relative surface area of the anode to the cathode impacts galvanic corrosion rates. It is found that the galvanic corrosion effect on carbon steel anode increases with the cathode/anode area ratios, and decreases with the increasing concentration of s2− in the solution. Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic. Galvanic Corrosion Cathode Anode Ratio.
From www.takomabattery.com
Design anode to cathode ratio of lithiumion battery The Best lithium Galvanic Corrosion Cathode Anode Ratio Another important factor in galvanic corrosion is the area effect or the ratio of cathodic to anodic area. The relative surface area of the anode to the cathode impacts galvanic corrosion rates. As a given amount of current flows in a galvanic couple,. The area ratio of the anode to cathode plays a dominant role in galvanic corrosion. Galvanic corrosion. Galvanic Corrosion Cathode Anode Ratio.