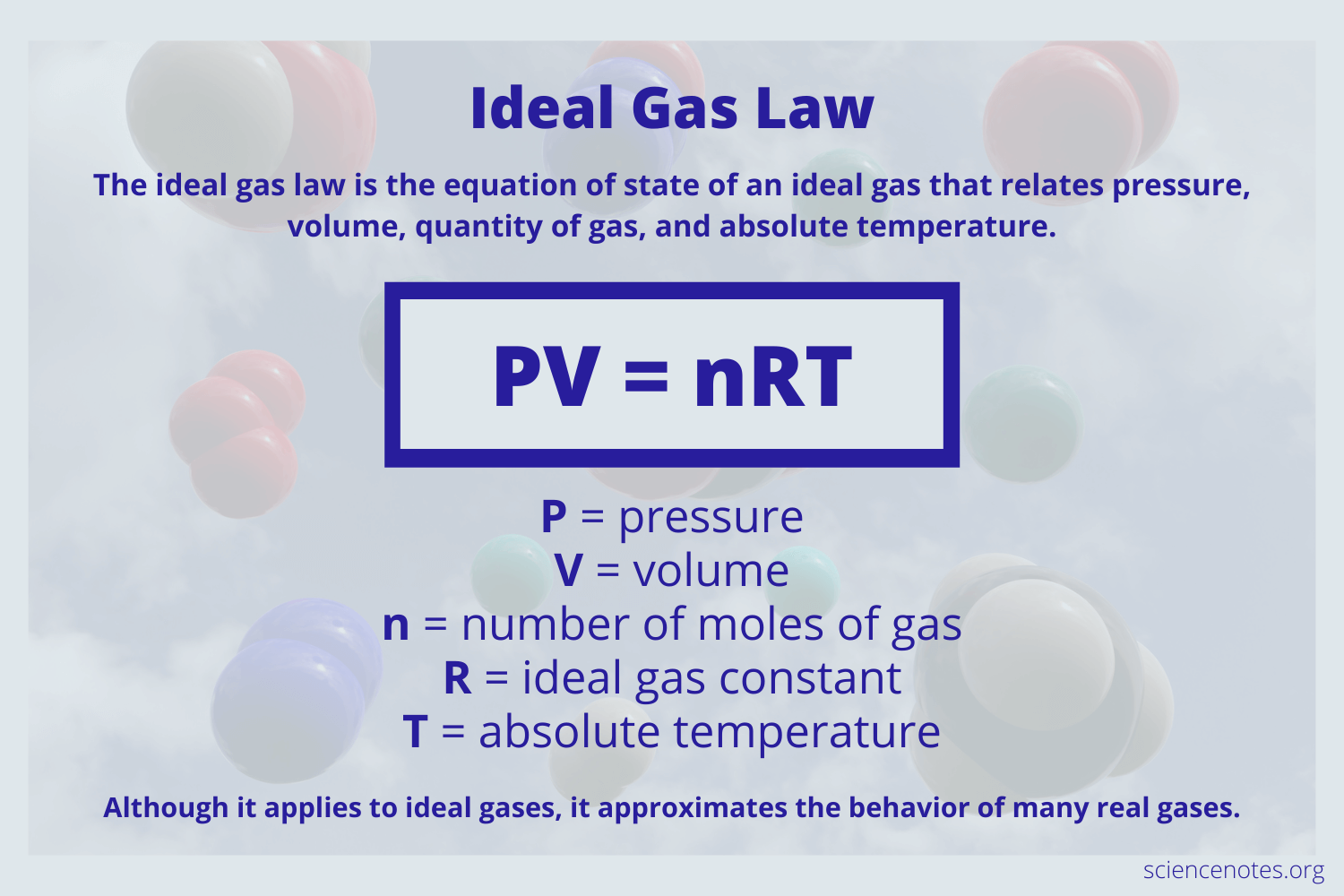
What S Another Word For Ideal Gas Law . The ideal gas law is very simply expressed: Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The temperature of the gas is proportional to the average kinetic energy of the molecules. And then two absolutely key assumptions, because. Synonyms for ideal gas law (other words and phrases for ideal gas law). It establishes a relationship between macroscopic properties like pressure, volume, temperature,. An ideal gas is suitable for modeling real gas and predicting the. An ideal gas is a hypothetical gas that is in close approximation to a real gas and obeys a specific set of laws. The ideal gas law is a law that explains the state of an ideal gas. In such a case, all gases obey an equation of state known as the ideal gas law: \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. Another way to say ideal gas law?
from inspiritvr.com
Synonyms for ideal gas law (other words and phrases for ideal gas law). An ideal gas is a hypothetical gas that is in close approximation to a real gas and obeys a specific set of laws. \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. The temperature of the gas is proportional to the average kinetic energy of the molecules. The ideal gas law is very simply expressed: An ideal gas is suitable for modeling real gas and predicting the. It establishes a relationship between macroscopic properties like pressure, volume, temperature,. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. Another way to say ideal gas law? And then two absolutely key assumptions, because.
Ideal Gas Law Study Guide Inspirit Learning Inc
What S Another Word For Ideal Gas Law The temperature of the gas is proportional to the average kinetic energy of the molecules. The ideal gas law is a law that explains the state of an ideal gas. It establishes a relationship between macroscopic properties like pressure, volume, temperature,. The temperature of the gas is proportional to the average kinetic energy of the molecules. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The ideal gas law is very simply expressed: An ideal gas is a hypothetical gas that is in close approximation to a real gas and obeys a specific set of laws. An ideal gas is suitable for modeling real gas and predicting the. In such a case, all gases obey an equation of state known as the ideal gas law: And then two absolutely key assumptions, because. \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. Synonyms for ideal gas law (other words and phrases for ideal gas law). Another way to say ideal gas law?
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal What S Another Word For Ideal Gas Law Another way to say ideal gas law? \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. And then two absolutely key assumptions, because. An ideal gas is suitable for modeling real gas and predicting the. The temperature of the gas is proportional to the average kinetic energy of the molecules. Ideal gas law, relation between. What S Another Word For Ideal Gas Law.
From www.e-streetlight.com
Ideal Gas Laws Worksheet E Street Light What S Another Word For Ideal Gas Law It establishes a relationship between macroscopic properties like pressure, volume, temperature,. Synonyms for ideal gas law (other words and phrases for ideal gas law). An ideal gas is suitable for modeling real gas and predicting the. The temperature of the gas is proportional to the average kinetic energy of the molecules. In such a case, all gases obey an equation. What S Another Word For Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID5758540 What S Another Word For Ideal Gas Law An ideal gas is suitable for modeling real gas and predicting the. It establishes a relationship between macroscopic properties like pressure, volume, temperature,. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each. What S Another Word For Ideal Gas Law.
From inspiritvr.com
Combined Gas Law Study Guide Inspirit Learning Inc What S Another Word For Ideal Gas Law In such a case, all gases obey an equation of state known as the ideal gas law: It establishes a relationship between macroscopic properties like pressure, volume, temperature,. An ideal gas is a hypothetical gas that is in close approximation to a real gas and obeys a specific set of laws. An ideal gas is suitable for modeling real gas. What S Another Word For Ideal Gas Law.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii What S Another Word For Ideal Gas Law Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The ideal gas law is very simply expressed: The temperature of the gas is proportional to the average kinetic energy of the. What S Another Word For Ideal Gas Law.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii What S Another Word For Ideal Gas Law And then two absolutely key assumptions, because. The ideal gas law is very simply expressed: In such a case, all gases obey an equation of state known as the ideal gas law: It establishes a relationship between macroscopic properties like pressure, volume, temperature,. The temperature of the gas is proportional to the average kinetic energy of the molecules. An ideal. What S Another Word For Ideal Gas Law.
From dokumen.tips
(PDF) Ideal Gas Law PPT DOKUMEN.TIPS What S Another Word For Ideal Gas Law \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. The temperature of the gas is proportional to the average kinetic energy of the molecules. The ideal gas law is very simply expressed: And then two absolutely key assumptions, because. Synonyms for ideal gas law (other words and phrases for ideal gas law). Another way to. What S Another Word For Ideal Gas Law.
From macblogtest.blogspot.com
Ideal Gas Law R Values PPT Gas Laws and Nature of Gases PowerPoint What S Another Word For Ideal Gas Law Synonyms for ideal gas law (other words and phrases for ideal gas law). And then two absolutely key assumptions, because. An ideal gas is suitable for modeling real gas and predicting the. In such a case, all gases obey an equation of state known as the ideal gas law: \ [ pv=nrt\] from which simpler gas laws such as boyle's,. What S Another Word For Ideal Gas Law.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID7067134 What S Another Word For Ideal Gas Law It establishes a relationship between macroscopic properties like pressure, volume, temperature,. In such a case, all gases obey an equation of state known as the ideal gas law: Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas. What S Another Word For Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 What S Another Word For Ideal Gas Law The temperature of the gas is proportional to the average kinetic energy of the molecules. In such a case, all gases obey an equation of state known as the ideal gas law: \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. It establishes a relationship between macroscopic properties like pressure, volume, temperature,. And then two. What S Another Word For Ideal Gas Law.
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal What S Another Word For Ideal Gas Law \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. An ideal gas is a hypothetical gas that. What S Another Word For Ideal Gas Law.
From www.youtube.com
Ideal Gas Law Practice Problems YouTube What S Another Word For Ideal Gas Law And then two absolutely key assumptions, because. \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. Another way to say ideal gas law? It establishes a relationship between macroscopic properties like pressure, volume, temperature,. An ideal gas is suitable for modeling real gas and predicting the. The temperature of the gas is proportional to the. What S Another Word For Ideal Gas Law.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net What S Another Word For Ideal Gas Law Another way to say ideal gas law? \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. An ideal gas is a hypothetical gas that is in close approximation to a real gas and obeys a specific set of laws. The ideal gas law is very simply expressed: An ideal gas is suitable for modeling real. What S Another Word For Ideal Gas Law.
From www.dreamstime.com
Ideal Gas Law Stock Illustrations 18 Ideal Gas Law Stock What S Another Word For Ideal Gas Law In such a case, all gases obey an equation of state known as the ideal gas law: Synonyms for ideal gas law (other words and phrases for ideal gas law). It establishes a relationship between macroscopic properties like pressure, volume, temperature,. An ideal gas is suitable for modeling real gas and predicting the. The temperature of the gas is proportional. What S Another Word For Ideal Gas Law.
From www.slideserve.com
PPT Thermal Physics (Thermodynamics) PowerPoint Presentation ID421070 What S Another Word For Ideal Gas Law Synonyms for ideal gas law (other words and phrases for ideal gas law). It establishes a relationship between macroscopic properties like pressure, volume, temperature,. The ideal gas law is very simply expressed: And then two absolutely key assumptions, because. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low. What S Another Word For Ideal Gas Law.
From www.slideserve.com
PPT The ideal gas law PowerPoint Presentation, free download ID3608170 What S Another Word For Ideal Gas Law An ideal gas is a hypothetical gas that is in close approximation to a real gas and obeys a specific set of laws. An ideal gas is suitable for modeling real gas and predicting the. It establishes a relationship between macroscopic properties like pressure, volume, temperature,. The ideal gas law is a law that explains the state of an ideal. What S Another Word For Ideal Gas Law.
From www.slideserve.com
PPT Basic Laws of Gases and Particulates PowerPoint Presentation What S Another Word For Ideal Gas Law And then two absolutely key assumptions, because. It establishes a relationship between macroscopic properties like pressure, volume, temperature,. The ideal gas law is very simply expressed: In such a case, all gases obey an equation of state known as the ideal gas law: Another way to say ideal gas law? \ [ pv=nrt\] from which simpler gas laws such as. What S Another Word For Ideal Gas Law.
From www.alamy.com
Ideal gas law, illustration Stock Photo Alamy What S Another Word For Ideal Gas Law An ideal gas is a hypothetical gas that is in close approximation to a real gas and obeys a specific set of laws. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each. What S Another Word For Ideal Gas Law.
From inspiritvr.com
Ideal Gas Law Study Guide Inspirit Learning Inc What S Another Word For Ideal Gas Law The temperature of the gas is proportional to the average kinetic energy of the molecules. An ideal gas is a hypothetical gas that is in close approximation to a real gas and obeys a specific set of laws. The ideal gas law is a law that explains the state of an ideal gas. The ideal gas law is very simply. What S Another Word For Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law 01 PowerPoint Presentation, free download ID What S Another Word For Ideal Gas Law An ideal gas is a hypothetical gas that is in close approximation to a real gas and obeys a specific set of laws. Synonyms for ideal gas law (other words and phrases for ideal gas law). It establishes a relationship between macroscopic properties like pressure, volume, temperature,. In such a case, all gases obey an equation of state known as. What S Another Word For Ideal Gas Law.
From www.slideserve.com
PPT C H A P T E R 14 The Ideal Gas Law and Theory PowerPoint What S Another Word For Ideal Gas Law \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. In such a case, all gases obey an equation of state known as the ideal gas law: It establishes a relationship between macroscopic properties like pressure, volume, temperature,. An ideal gas is a hypothetical gas that is in close approximation to a real gas and obeys. What S Another Word For Ideal Gas Law.
From www.britannica.com
perfect gas law chemistry and physics Britannica What S Another Word For Ideal Gas Law It establishes a relationship between macroscopic properties like pressure, volume, temperature,. The ideal gas law is very simply expressed: An ideal gas is a hypothetical gas that is in close approximation to a real gas and obeys a specific set of laws. Another way to say ideal gas law? Ideal gas law, relation between the pressure p, volume v, and. What S Another Word For Ideal Gas Law.
From brainly.com
Which equation agrees with the ideal gas law? What S Another Word For Ideal Gas Law An ideal gas is a hypothetical gas that is in close approximation to a real gas and obeys a specific set of laws. The temperature of the gas is proportional to the average kinetic energy of the molecules. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures. What S Another Word For Ideal Gas Law.
From www.slideserve.com
PPT using The Ideal Gas Law PowerPoint Presentation, free download What S Another Word For Ideal Gas Law The ideal gas law is very simply expressed: An ideal gas is a hypothetical gas that is in close approximation to a real gas and obeys a specific set of laws. In such a case, all gases obey an equation of state known as the ideal gas law: The temperature of the gas is proportional to the average kinetic energy. What S Another Word For Ideal Gas Law.
From millingschem.com
Chemistry Resources 3 MillingsChem What S Another Word For Ideal Gas Law An ideal gas is a hypothetical gas that is in close approximation to a real gas and obeys a specific set of laws. The ideal gas law is very simply expressed: In such a case, all gases obey an equation of state known as the ideal gas law: Another way to say ideal gas law? Synonyms for ideal gas law. What S Another Word For Ideal Gas Law.
From byjus.com
Ideal Gas Law Equation Compressibility Of Natural Gas Chemistry What S Another Word For Ideal Gas Law \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. An ideal gas is suitable for modeling real gas and predicting the. The ideal gas law is very simply expressed: The temperature of the gas is proportional to the average kinetic energy of the molecules. An ideal gas is a hypothetical gas that is in close. What S Another Word For Ideal Gas Law.
From www.slideserve.com
PPT Chapter 13 Section 13.2 The Ideal Gas Law PowerPoint Presentation What S Another Word For Ideal Gas Law The ideal gas law is very simply expressed: Another way to say ideal gas law? \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. And then two absolutely key assumptions, because. The ideal gas law is a law that explains the state of an ideal gas. An ideal gas is suitable for modeling real gas. What S Another Word For Ideal Gas Law.
From www.pinterest.com
Combined Gas Law Definition, Formula, Examples Boyle's law, Ideal What S Another Word For Ideal Gas Law An ideal gas is suitable for modeling real gas and predicting the. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. The ideal gas law is very simply expressed: An ideal. What S Another Word For Ideal Gas Law.
From studylib.net
Ideal Gas Law What S Another Word For Ideal Gas Law Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. It establishes a relationship between macroscopic properties like pressure, volume, temperature,. An ideal gas is suitable for modeling real gas and predicting. What S Another Word For Ideal Gas Law.
From www.studocu.com
Ideal Gas Law Worksheet 2 Answer Ideal Gas Law Worksheet PV = nRT Use What S Another Word For Ideal Gas Law The ideal gas law is very simply expressed: Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. Another way to say ideal gas law? And then two absolutely key assumptions, because.. What S Another Word For Ideal Gas Law.
From mmerevise.co.uk
The Ideal Gas Equation MME What S Another Word For Ideal Gas Law The temperature of the gas is proportional to the average kinetic energy of the molecules. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. An ideal gas is a hypothetical gas. What S Another Word For Ideal Gas Law.
From www.vecteezy.com
Ideal Gas Law formula. Boyle law, charles law, combined law vector What S Another Word For Ideal Gas Law Synonyms for ideal gas law (other words and phrases for ideal gas law). In such a case, all gases obey an equation of state known as the ideal gas law: The ideal gas law is a law that explains the state of an ideal gas. Another way to say ideal gas law? An ideal gas is suitable for modeling real. What S Another Word For Ideal Gas Law.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii What S Another Word For Ideal Gas Law The temperature of the gas is proportional to the average kinetic energy of the molecules. And then two absolutely key assumptions, because. Synonyms for ideal gas law (other words and phrases for ideal gas law). Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures,. What S Another Word For Ideal Gas Law.
From atomstalk.com
The Gas Laws AtomsTalk What S Another Word For Ideal Gas Law And then two absolutely key assumptions, because. An ideal gas is a hypothetical gas that is in close approximation to a real gas and obeys a specific set of laws. \ [ pv=nrt\] from which simpler gas laws such as boyle's, charles's, avogadro's and. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas. What S Another Word For Ideal Gas Law.
From slideplayer.com
The Ideal Gas Law and Theory ppt download What S Another Word For Ideal Gas Law Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. It establishes a relationship between macroscopic properties like pressure, volume, temperature,. In such a case, all gases obey an equation of state. What S Another Word For Ideal Gas Law.