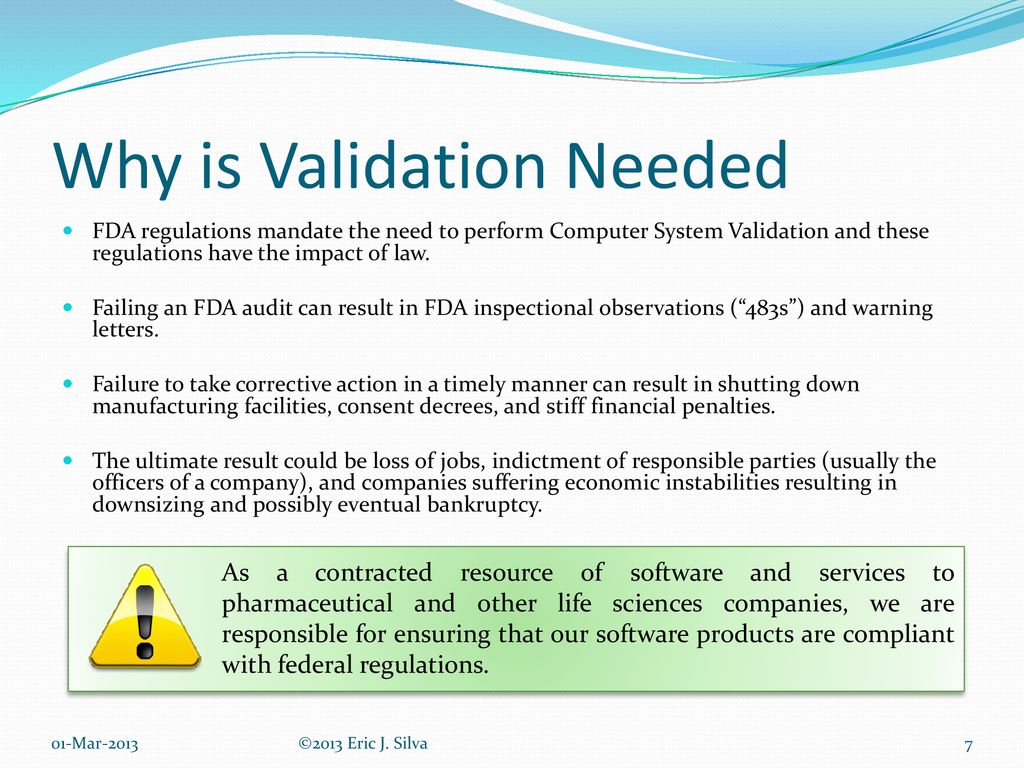
Computer System Validation Definition As Per Fda . This guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does. Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. Learn more about the fda’s new approach to computer software validation and how the benefits can improve manufacturer compliance and quality. Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical.
from slideplayer.com
This guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the. Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. Learn more about the fda’s new approach to computer software validation and how the benefits can improve manufacturer compliance and quality. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does. Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized.
Computer System Validation ppt download
Computer System Validation Definition As Per Fda Learn more about the fda’s new approach to computer software validation and how the benefits can improve manufacturer compliance and quality. Learn more about the fda’s new approach to computer software validation and how the benefits can improve manufacturer compliance and quality. Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. This guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical.
From www.greenlight.guru
FDA Computer System & Software Validation What You’ve Known For 20 Computer System Validation Definition As Per Fda This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. This guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the. Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. Learn more about the. Computer System Validation Definition As Per Fda.
From www.greenlight.guru
FDA Computer System & Software Validation What You’ve Known For 20 Computer System Validation Definition As Per Fda Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does. Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit. Computer System Validation Definition As Per Fda.
From www.guerraconsultinggroup.com
FDA Computer System Validation Certification Program Computer System Validation Definition As Per Fda This guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the. Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to. Computer System Validation Definition As Per Fda.
From www.pinterest.com
Testing for Compliant Computer Systems for FDA fda validation FMLA Computer System Validation Definition As Per Fda Learn more about the fda’s new approach to computer software validation and how the benefits can improve manufacturer compliance and quality. This guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. Computer software. Computer System Validation Definition As Per Fda.
From issuu.com
The Scope of Pharma Computer System Validation by Company Connect Computer System Validation Definition As Per Fda Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. Learn more about the fda’s new approach to computer software validation and how the benefits can improve manufacturer compliance and quality. Computer software. Computer System Validation Definition As Per Fda.
From www.spkaa.com
FDA Part 11 Your Guide to Computer System Validation CSV Compliance Computer System Validation Definition As Per Fda Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does. Learn more about the fda’s new approach to computer software validation and how the benefits can improve. Computer System Validation Definition As Per Fda.
From www.mmsholdings.com
Crucial Steps In Computer System Validation For FDA Approval Computer System Validation Definition As Per Fda Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. Learn more about the fda’s new approach to computer software validation and how the benefits can improve manufacturer compliance and quality. Computer systems. Computer System Validation Definition As Per Fda.
From www.spkaa.com
How To Complete Computer Systems Validation (FDA) SPK and Associates Computer System Validation Definition As Per Fda Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. This guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the. Computerized systems validation is the documented proof enabling to conclude with a high degree of. Computer System Validation Definition As Per Fda.
From validationcenter.com
What is Computer System Validation and How Do You Do It? Computer System Validation Definition As Per Fda This guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does. Learn more about the fda’s new approach to computer software validation and how the benefits can improve. Computer System Validation Definition As Per Fda.
From www.slideserve.com
PPT FDA Computer System Validation Steps PowerPoint Presentation Computer System Validation Definition As Per Fda Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. Learn more about the fda’s new approach to computer software validation and how the benefits can improve manufacturer compliance and quality. Computer systems validation (csv) is a documented process that is required by regulatory. Computer System Validation Definition As Per Fda.
From www.totalpharmaceuticaltopics.com
Computer system validation (CSV) in Pharmaceutical Industry !! Introduction Computer System Validation Definition As Per Fda This guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the. Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve,. Computer System Validation Definition As Per Fda.
From www.getreskilled.com
What is Computer System Validation (CSV) in Pharma? Computer System Validation Definition As Per Fda This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does. Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified. Computer System Validation Definition As Per Fda.
From www.k2c.com
Computerised System Validation Computer System Validation Definition As Per Fda Learn more about the fda’s new approach to computer software validation and how the benefits can improve manufacturer compliance and quality. Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. This document addresses issues pertaining to computerized systems used to create, modify, maintain,. Computer System Validation Definition As Per Fda.
From kvalito.ch
RiskBased Computerized System Validation (CSV) and Computer Software Computer System Validation Definition As Per Fda Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does. Learn more about the fda’s new approach to computer software validation and how the benefits can improve. Computer System Validation Definition As Per Fda.
From www.greenlight.guru
FDA Computer System & Software Validation What You’ve Known For 20 Computer System Validation Definition As Per Fda This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. This guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the. Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. Computer software assurance (csa). Computer System Validation Definition As Per Fda.
From dokumen.tips
(PDF) Applying Risk Management to Computer System Validation Computer System Validation Definition As Per Fda Learn more about the fda’s new approach to computer software validation and how the benefits can improve manufacturer compliance and quality. Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. Computerized systems validation is the documented proof enabling to conclude with a high. Computer System Validation Definition As Per Fda.
From slcontrols.com
Infographic Computer System Validation Vs. Computer Software Assurance Computer System Validation Definition As Per Fda This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the. Computer System Validation Definition As Per Fda.
From www.vrogue.co
Ppt Fda Computer System Validation Steps Powerpoint P vrogue.co Computer System Validation Definition As Per Fda This guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the. Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized. Computer System Validation Definition As Per Fda.
From www.bioprocessonline.com
Are You Ready FDA’s Transition From Computer System Validation To Computer System Validation Definition As Per Fda Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a. Computer System Validation Definition As Per Fda.
From www.vrogue.co
Ppt Fda Computer System Validation Steps Powerpoint P vrogue.co Computer System Validation Definition As Per Fda Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. Learn more about the fda’s new approach to computer software validation and how the benefits can. Computer System Validation Definition As Per Fda.
From www.greenlight.guru
FDA Computer System & Software Validation What You’ve Known For 20 Computer System Validation Definition As Per Fda Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does. This guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the. Learn more about the fda’s new approach to computer software validation and how the benefits can improve. Computer System Validation Definition As Per Fda.
From www.slideserve.com
PPT Computer System Validation Compliance Required by FDA PowerPoint Computer System Validation Definition As Per Fda Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does. Computerized systems validation is the documented proof enabling to conclude. Computer System Validation Definition As Per Fda.
From www.linkedin.com
COMPUTER SYSTEM VALIDATION Computer System Validation Definition As Per Fda Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. Learn more about the fda’s new approach to computer software validation and how the benefits can. Computer System Validation Definition As Per Fda.
From www.slideserve.com
PPT FDA Computer System Validation Steps PowerPoint Presentation Computer System Validation Definition As Per Fda Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does. Learn more about the fda’s new approach to computer software validation and how the benefits can improve manufacturer compliance and quality. Computer software assurance (csa) is a case for quality initiative that arose when computer systems. Computer System Validation Definition As Per Fda.
From www.spkaa.com
Computer Systems Validation How To Avoid FDA Warning Letters C.F.R Computer System Validation Definition As Per Fda Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does. This guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable. Computer System Validation Definition As Per Fda.
From www.greenlight.guru
FDA Computer System & Software Validation What You’ve Known For 20 Computer System Validation Definition As Per Fda Learn more about the fda’s new approach to computer software validation and how the benefits can improve manufacturer compliance and quality. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier. Computer System Validation Definition As Per Fda.
From fivevalidation.com
What is Computer System Validation? Computer System Validation Definition As Per Fda Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit. Computer System Validation Definition As Per Fda.
From slideplayer.com
Computer System Validation ppt download Computer System Validation Definition As Per Fda Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. Learn more about the fda’s new approach to computer software validation and how the benefits can improve manufacturer compliance and quality. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. This guidance. Computer System Validation Definition As Per Fda.
From www.datacor.com
2024 FDA Software Validation Example Template, Checklist & Process Computer System Validation Definition As Per Fda Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. Computer systems validation (csv) is a documented process that is required by regulatory agencies around. Computer System Validation Definition As Per Fda.
From veeprho.com
Computer System Validation in Pharmaceutical Industry Veeprho Computer System Validation Definition As Per Fda This guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does. Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that. Computer System Validation Definition As Per Fda.
From clickon24.com
What is Computer System Validation? A Quick Guide Matrix Servers Computer System Validation Definition As Per Fda Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. Learn more about the fda’s new approach to computer software validation and how the benefits can. Computer System Validation Definition As Per Fda.
From www.slideserve.com
PPT Computer System Validation in Pharmaceuticals PowerPoint Computer System Validation Definition As Per Fda Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. This guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized. Computer System Validation Definition As Per Fda.
From bncengineers.com
Computer system validation Industrial Automation Computer System Validation Definition As Per Fda Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance. Computer System Validation Definition As Per Fda.
From vdocuments.site
Computer System Validation Computer System Validation Definition As Per Fda Learn more about the fda’s new approach to computer software validation and how the benefits can improve manufacturer compliance and quality. This document addresses issues pertaining to computerized systems used to create, modify, maintain, archive, retrieve, or transmit clinical. Computerized systems validation is the documented proof enabling to conclude with a high degree of assurance that a computerized. Computer systems. Computer System Validation Definition As Per Fda.
From www.greenlight.guru
FDA Computer System & Software Validation What You’ve Known For 20 Computer System Validation Definition As Per Fda Computer software assurance (csa) is a case for quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization. Learn more about the fda’s new approach to computer software validation and how the benefits can improve manufacturer compliance and quality. Computer systems validation (csv) is a documented process that is required by regulatory. Computer System Validation Definition As Per Fda.