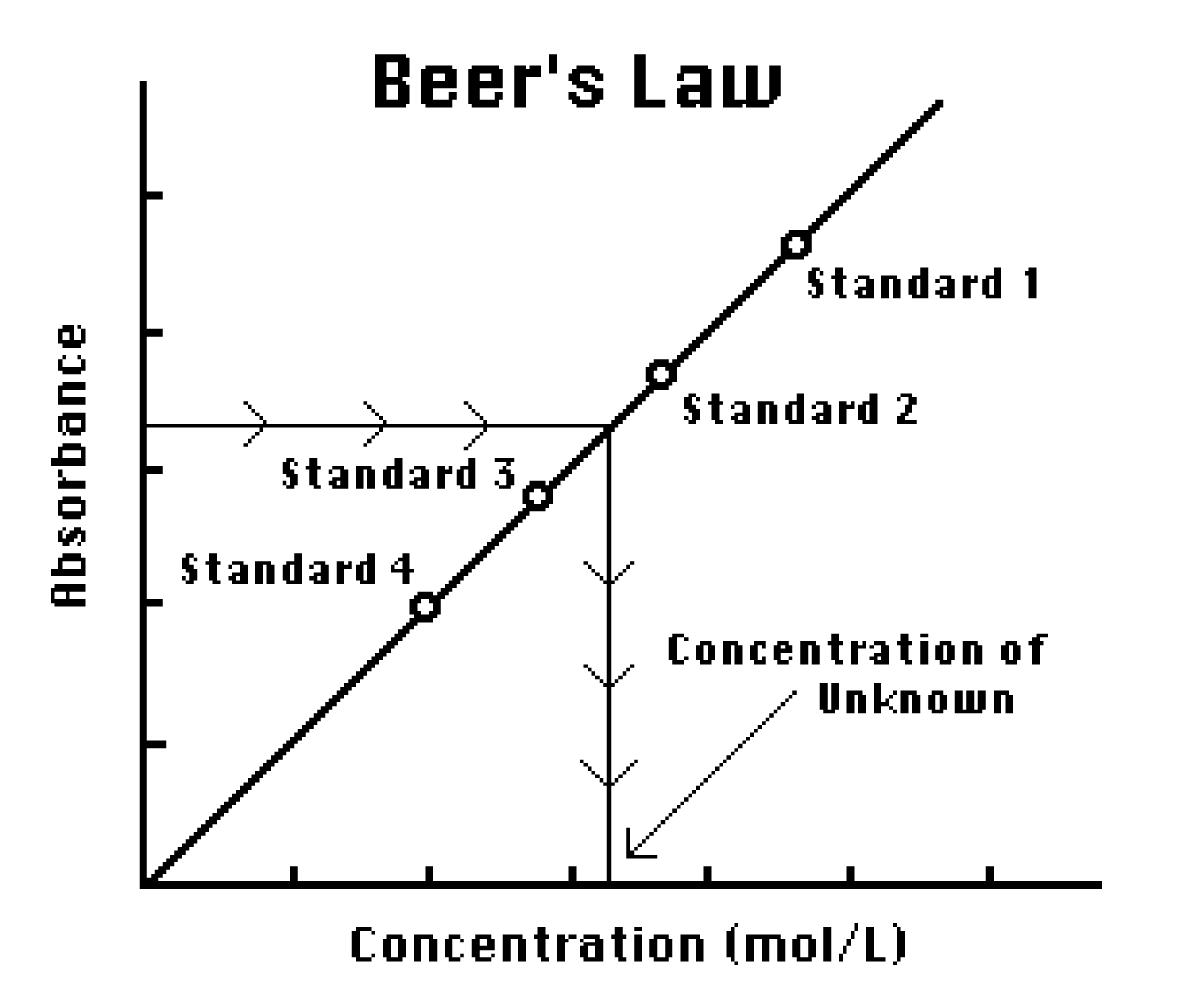
Beer Lambert Law Calibration Curve . finding concentration by plotting a calibration curve. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Calibration curves based on beer’s law are common in quantitative analyses. calibration curves based on beer’s law are common in quantitative analyses. As is often the case, the. Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure drug and marketed tablet formulation consisting in. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law.
from www.vernier.com
finding concentration by plotting a calibration curve. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. As is often the case, the. Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure drug and marketed tablet formulation consisting in. calibration curves based on beer’s law are common in quantitative analyses. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Calibration curves based on beer’s law are common in quantitative analyses.
Determining the Concentration of a Solution Beer's Law > Experiment 17
Beer Lambert Law Calibration Curve As is often the case, the. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. As is often the case, the. finding concentration by plotting a calibration curve. calibration curves based on beer’s law are common in quantitative analyses. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure drug and marketed tablet formulation consisting in. Calibration curves based on beer’s law are common in quantitative analyses. Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law.
From chem.libretexts.org
8.2 Beer's Law Chemistry LibreTexts Beer Lambert Law Calibration Curve calibration curves based on beer’s law are common in quantitative analyses. Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. As is often the case, the. the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure drug and marketed tablet. Beer Lambert Law Calibration Curve.
From www.researchgate.net
Calibration curve according to BeerLambert equation for tetracycline Beer Lambert Law Calibration Curve As is often the case, the. calibration curves based on beer’s law are common in quantitative analyses. finding concentration by plotting a calibration curve. Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. since the concentration, path length and molar absorptivity are all directly proportional to. Beer Lambert Law Calibration Curve.
From www.researchgate.net
Comparison of the typical calibration curves predicted by the PDE and Beer Lambert Law Calibration Curve Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to. Beer Lambert Law Calibration Curve.
From www.wam.umd.edu
Simulation of Instrumental Deviation from Beer's Law Beer Lambert Law Calibration Curve Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure drug and marketed tablet formulation consisting in. Calibration curves based on beer’s law are common in quantitative analyses. It provides a mathematical. Beer Lambert Law Calibration Curve.
From www.researchgate.net
Calibration curves for different concentrations of ABTS ox and Beer Lambert Law Calibration Curve As is often the case, the. calibration curves based on beer’s law are common in quantitative analyses. Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure drug and marketed tablet. Beer Lambert Law Calibration Curve.
From royalsocietypublishing.org
Misuse of BeerLambert Law and other calibration curves Royal Society Beer Lambert Law Calibration Curve equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Calibration curves based on beer’s law are common in quantitative analyses. Doing it this way you. Beer Lambert Law Calibration Curve.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer Lambert Law Calibration Curve Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. calibration curves based on beer’s law are common in quantitative analyses. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. since the concentration, path length and molar absorptivity are. Beer Lambert Law Calibration Curve.
From thechemistrynotes.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law Calibration Curve As is often the case, the. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. since the concentration, path length and molar absorptivity are all directly proportional to. Beer Lambert Law Calibration Curve.
From www.savemyexams.com
BeerLambert Law College Board AP Chemistry Revision Notes 2022 Beer Lambert Law Calibration Curve It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure. Beer Lambert Law Calibration Curve.
From www.youtube.com
A.8.6 Find the concentration of a solution via calibration curve (Beer Beer Lambert Law Calibration Curve the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure drug and marketed tablet formulation consisting in. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. finding concentration by plotting a calibration curve. calibration curves based on beer’s law. Beer Lambert Law Calibration Curve.
From royalsocietypublishing.org
Misuse of BeerLambert Law and other calibration curves Royal Society Beer Lambert Law Calibration Curve As is often the case, the. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Calibration curves based on beer’s law are common in quantitative analyses. . Beer Lambert Law Calibration Curve.
From www.researchgate.net
Fig. S3. Beer's law calibration curve for the UVVis spectrum of Beer Lambert Law Calibration Curve calibration curves based on beer’s law are common in quantitative analyses. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. As is often the case, the. the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure drug and marketed tablet formulation consisting. Beer Lambert Law Calibration Curve.
From royalsocietypublishing.org
Misuse of BeerLambert Law and other calibration curves Royal Society Beer Lambert Law Calibration Curve since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure. Beer Lambert Law Calibration Curve.
From www.researchgate.net
Fluid calibration. The calibration curve, which is linear thereby Beer Lambert Law Calibration Curve Calibration curves based on beer’s law are common in quantitative analyses. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. calibration curves based on beer’s law are common in quantitative analyses. the authors of carry out an experiment to introduce a method to. Beer Lambert Law Calibration Curve.
From www.numerade.com
SOLVED Given the following calibration curve for solution Beer Beer Lambert Law Calibration Curve Calibration curves based on beer’s law are common in quantitative analyses. finding concentration by plotting a calibration curve. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Doing it this way you don't have to rely on a value of molar absorptivity, the reliability. Beer Lambert Law Calibration Curve.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer Lambert Law Calibration Curve finding concentration by plotting a calibration curve. Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Calibration curves based on beer’s law are common in quantitative analyses. equation 8.2.3. Beer Lambert Law Calibration Curve.
From www.numerade.com
SOLVED Given the following calibration curve for solution X Beer Beer Lambert Law Calibration Curve calibration curves based on beer’s law are common in quantitative analyses. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. finding concentration by plotting a calibration curve. the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure drug and. Beer Lambert Law Calibration Curve.
From www.researchgate.net
Fig. S1. Beer's law calibration curve for the UVVis spectrum of Beer Lambert Law Calibration Curve Calibration curves based on beer’s law are common in quantitative analyses. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. finding concentration by plotting a calibration curve. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. calibration curves. Beer Lambert Law Calibration Curve.
From exoxwjcgv.blob.core.windows.net
Beer's Law Graph Maker at Paul Bernstein blog Beer Lambert Law Calibration Curve It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. calibration curves based on beer’s law are common in quantitative analyses. Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. the authors of carry out an experiment to introduce. Beer Lambert Law Calibration Curve.
From www.researchgate.net
Fig. S2. Beer's law calibration curve for the UVVis spectrum of Beer Lambert Law Calibration Curve calibration curves based on beer’s law are common in quantitative analyses. Calibration curves based on beer’s law are common in quantitative analyses. As is often the case, the. Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. finding concentration by plotting a calibration curve. It provides a. Beer Lambert Law Calibration Curve.
From www.savemyexams.com
BeerLambert Law College Board AP Chemistry Revision Notes 2022 Beer Lambert Law Calibration Curve It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. finding concentration by plotting a calibration curve. As is often the case, the. the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure drug and marketed tablet formulation consisting in. equation 8.2.3. Beer Lambert Law Calibration Curve.
From mckennaroscervantes.blogspot.com
Beer's Lambert Law Equation MckennarosCervantes Beer Lambert Law Calibration Curve Calibration curves based on beer’s law are common in quantitative analyses. finding concentration by plotting a calibration curve. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. the authors. Beer Lambert Law Calibration Curve.
From www.researchgate.net
Fig. S2. Beer's law calibration curve for the UVVis spectrum of Beer Lambert Law Calibration Curve As is often the case, the. finding concentration by plotting a calibration curve. calibration curves based on beer’s law are common in quantitative analyses. Calibration curves based on beer’s law are common in quantitative analyses. the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure drug and marketed tablet formulation. Beer Lambert Law Calibration Curve.
From www.numerade.com
SOLVED 20 pts Knowing that Beer's law is A = kC derived from the Beer Beer Lambert Law Calibration Curve Calibration curves based on beer’s law are common in quantitative analyses. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. equation 8.2.3 and equation. Beer Lambert Law Calibration Curve.
From www.chegg.com
Solved Given the absorbance and concentration data from Beer Lambert Law Calibration Curve Calibration curves based on beer’s law are common in quantitative analyses. calibration curves based on beer’s law are common in quantitative analyses. Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known. Beer Lambert Law Calibration Curve.
From www.purechemistry.org
BeerLambert Law Purechemistry Beer Lambert Law Calibration Curve Calibration curves based on beer’s law are common in quantitative analyses. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. calibration curves based on beer’s law are common in quantitative analyses. As is often the case, the. It provides a mathematical relationship between the. Beer Lambert Law Calibration Curve.
From www.studypool.com
SOLUTION Beer lambert calibration curve Studypool Beer Lambert Law Calibration Curve Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. calibration curves based on beer’s law are common in quantitative analyses. Calibration curves based on beer’s law are common in quantitative analyses. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known. Beer Lambert Law Calibration Curve.
From www.chegg.com
Solved The BeerLambert Law (A=εlc) is used to plot a Beer Lambert Law Calibration Curve since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. calibration curves based on beer’s law are common in quantitative analyses. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Calibration curves based on beer’s law. Beer Lambert Law Calibration Curve.
From royalsocietypublishing.org
Misuse of BeerLambert Law and other calibration curves Royal Society Beer Lambert Law Calibration Curve equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure drug and marketed tablet formulation consisting in. finding concentration by plotting a calibration curve. As is often the case, the. Doing. Beer Lambert Law Calibration Curve.
From www.researchgate.net
Fig. S3. Beer's law calibration curve for the UVVis spectrum of Beer Lambert Law Calibration Curve equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure drug and marketed tablet formulation consisting in. since the concentration, path length and molar absorptivity are all directly proportional to the. Beer Lambert Law Calibration Curve.
From stuff.iorodeo.com
Lab 2 Beer’s Law and Molar Extinction Coefficients — Colorimeter User Beer Lambert Law Calibration Curve equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. finding concentration by plotting a calibration curve. calibration curves based on beer’s law are common in quantitative analyses. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the. Beer Lambert Law Calibration Curve.
From www.researchgate.net
Fig. S2. Beer's law calibration curve for the UVVis spectrum of Beer Lambert Law Calibration Curve calibration curves based on beer’s law are common in quantitative analyses. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. equation 8.2.3 and equation 8.2.4,. Beer Lambert Law Calibration Curve.
From www.researchgate.net
Calibration curve according to BeerLambert equation for tetracycline Beer Lambert Law Calibration Curve As is often the case, the. calibration curves based on beer’s law are common in quantitative analyses. Doing it this way you don't have to rely on a value of molar absorptivity, the reliability of the beer. finding concentration by plotting a calibration curve. Calibration curves based on beer’s law are common in quantitative analyses. since the. Beer Lambert Law Calibration Curve.
From chem.libretexts.org
10.2 Spectroscopy Based on Absorption Chemistry LibreTexts Beer Lambert Law Calibration Curve the authors of carry out an experiment to introduce a method to estimate the amlopidine in pure drug and marketed tablet formulation consisting in. Calibration curves based on beer’s law are common in quantitative analyses. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. finding concentration by plotting a. Beer Lambert Law Calibration Curve.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer Lambert Law Calibration Curve equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. calibration curves based on beer’s law are common in quantitative analyses. the authors of. Beer Lambert Law Calibration Curve.