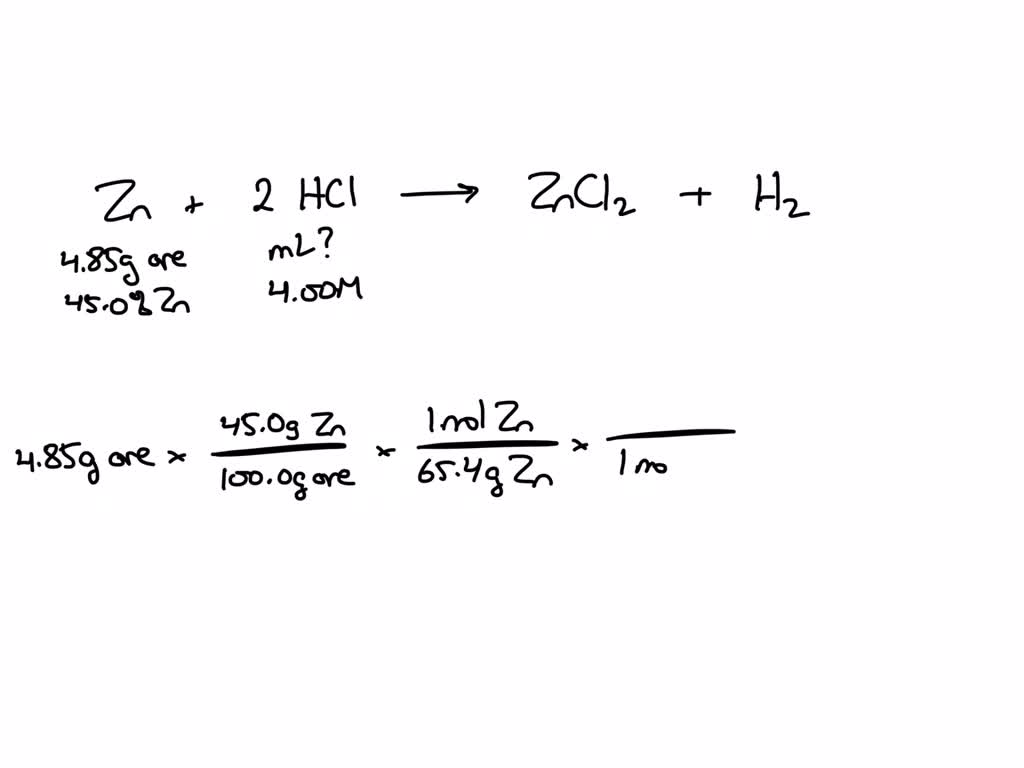Zinc Reacts With Hydrochloric Acid Formula . reaction of zinc and hydrochloric acid. in this video, we'll be exploring the reaction between zinc metal and hydrochloric acid. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. zinc metal reacts with hydrochloric acid according to the balanced equation: zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g. zinc metal reacts with hydrochloric acid according to the balanced equation: Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. Let’s examine the example of the interaction between zinc and hydrochloric acid.
from www.numerade.com
zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. in this video, we'll be exploring the reaction between zinc metal and hydrochloric acid. Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. reaction of zinc and hydrochloric acid. zinc metal reacts with hydrochloric acid according to the balanced equation: Let’s examine the example of the interaction between zinc and hydrochloric acid. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. zinc metal reacts with hydrochloric acid according to the balanced equation:
SOLVED Zinc reacts with hydrochloric acid according to the reaction
Zinc Reacts With Hydrochloric Acid Formula this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. zinc metal reacts with hydrochloric acid according to the balanced equation: Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g. reaction of zinc and hydrochloric acid. Let’s examine the example of the interaction between zinc and hydrochloric acid. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. in this video, we'll be exploring the reaction between zinc metal and hydrochloric acid. zinc metal reacts with hydrochloric acid according to the balanced equation: for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas.
From www.coursehero.com
[Solved] Zinc reacts with hydrochloric acid according to the reaction Zinc Reacts With Hydrochloric Acid Formula reaction of zinc and hydrochloric acid. zinc metal reacts with hydrochloric acid according to the balanced equation: this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. zinc metal reacts with hydrochloric acid according to the balanced equation: Let’s examine the example of the interaction between zinc and hydrochloric. Zinc Reacts With Hydrochloric Acid Formula.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Zinc Reacts With Hydrochloric Acid Formula zinc metal reacts with hydrochloric acid according to the balanced equation: this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Let’s examine the example of the. Zinc Reacts With Hydrochloric Acid Formula.
From www.alamy.com
Reaction of zinc with hydrochloric acid Stock Photo Alamy Zinc Reacts With Hydrochloric Acid Formula Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g. reaction of zinc and hydrochloric acid. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where. Zinc Reacts With Hydrochloric Acid Formula.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube Zinc Reacts With Hydrochloric Acid Formula Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g. zinc metal reacts with hydrochloric acid according to the balanced equation: . Zinc Reacts With Hydrochloric Acid Formula.
From www.chegg.com
Solved 1. Zinc metal reacts with hydrochloric acid according Zinc Reacts With Hydrochloric Acid Formula Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. zinc metal reacts with hydrochloric acid according to the balanced equation: Let’s examine the example of the interaction between zinc and hydrochloric. Zinc Reacts With Hydrochloric Acid Formula.
From www.numerade.com
SOLVEDZinc metal reacts with hydrochloric acid according to the Zinc Reacts With Hydrochloric Acid Formula in this video, we'll be exploring the reaction between zinc metal and hydrochloric acid. Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. zinc metal reacts with hydrochloric acid according to the balanced equation: zinc. Zinc Reacts With Hydrochloric Acid Formula.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc Reacts With Hydrochloric Acid Formula zinc metal reacts with hydrochloric acid according to the balanced equation: Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. reaction of zinc and hydrochloric acid. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. Let’s examine the example of the interaction between zinc and. Zinc Reacts With Hydrochloric Acid Formula.
From questions.kunduz.com
Zinc reacts with hydrochloric acid accord... Physical Chemistry Zinc Reacts With Hydrochloric Acid Formula zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. reaction of zinc and hydrochloric acid. zinc metal reacts with hydrochloric acid according to the balanced equation: zinc metal reacts with hydrochloric acid according to the balanced equation: Let’s examine the example of. Zinc Reacts With Hydrochloric Acid Formula.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Zinc Reacts With Hydrochloric Acid Formula zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. in this video, we'll be exploring the reaction between zinc metal and hydrochloric acid. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2(. Zinc Reacts With Hydrochloric Acid Formula.
From www.solutioninn.com
[Solved] 2. Zinc reacts with hydrochloric acid acc SolutionInn Zinc Reacts With Hydrochloric Acid Formula Let’s examine the example of the interaction between zinc and hydrochloric acid. Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. reaction. Zinc Reacts With Hydrochloric Acid Formula.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc Reacts With Hydrochloric Acid Formula Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g. Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. Let’s examine the example of the interaction between zinc and hydrochloric acid. zinc metal reacts with hydrochloric acid according to the balanced equation: zn + hcl = zncl2 + h2 is a single displacement (substitution). Zinc Reacts With Hydrochloric Acid Formula.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc Reacts With Hydrochloric Acid Formula for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Let’s examine the example of the interaction between zinc and hydrochloric acid. zinc metal reacts with hydrochloric acid according to. Zinc Reacts With Hydrochloric Acid Formula.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc Reacts With Hydrochloric Acid Formula reaction of zinc and hydrochloric acid. in this video, we'll be exploring the reaction between zinc metal and hydrochloric acid. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. . Zinc Reacts With Hydrochloric Acid Formula.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0309 Science Zinc Reacts With Hydrochloric Acid Formula Let’s examine the example of the interaction between zinc and hydrochloric acid. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. zinc metal reacts with hydrochloric acid according to the balanced equation: for example, zinc metal reacts with hydrochloric acid, producing zinc chloride. Zinc Reacts With Hydrochloric Acid Formula.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Zinc Reacts With Hydrochloric Acid Formula this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. Let’s examine the example of the interaction between zinc and hydrochloric acid. in this video, we'll be exploring the reaction between zinc metal and hydrochloric acid. zinc metal reacts with hydrochloric acid according to the balanced equation: zinc metal. Zinc Reacts With Hydrochloric Acid Formula.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc Reacts With Hydrochloric Acid Formula this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. reaction of zinc and hydrochloric acid. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Let’s. Zinc Reacts With Hydrochloric Acid Formula.
From www.chegg.com
Solved aboratory 5 .. a. Reaction of zinc with hydrochloric Zinc Reacts With Hydrochloric Acid Formula Let’s examine the example of the interaction between zinc and hydrochloric acid. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g. Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. zinc metal reacts with hydrochloric acid according to the balanced equation: zinc metal reacts with hydrochloric acid according to the balanced equation: . Zinc Reacts With Hydrochloric Acid Formula.
From www.youtube.com
Reaction Of Zinc with Hydrochloric acid Chemistry demonstration YouTube Zinc Reacts With Hydrochloric Acid Formula zinc metal reacts with hydrochloric acid according to the balanced equation: Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. for example, zinc metal reacts with hydrochloric acid, producing zinc. Zinc Reacts With Hydrochloric Acid Formula.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc Reacts With Hydrochloric Acid Formula in this video, we'll be exploring the reaction between zinc metal and hydrochloric acid. zinc metal reacts with hydrochloric acid according to the balanced equation: Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. reaction of zinc and hydrochloric acid. zn + hcl = zncl2 + h2 is a single displacement (substitution). Zinc Reacts With Hydrochloric Acid Formula.
From www.numerade.com
SOLVED Zinc reacts with hydrochloric acid according to the reaction Zinc Reacts With Hydrochloric Acid Formula reaction of zinc and hydrochloric acid. in this video, we'll be exploring the reaction between zinc metal and hydrochloric acid. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. zinc metal reacts with. Zinc Reacts With Hydrochloric Acid Formula.
From www.chegg.com
Solved Zinc metal reacts with aqueous hydrochloric acid to Zinc Reacts With Hydrochloric Acid Formula this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. zinc metal reacts with hydrochloric acid according to the balanced equation: Let’s examine the example of the interaction between zinc and hydrochloric acid. reaction of zinc and hydrochloric acid. for example, zinc metal reacts with hydrochloric acid, producing zinc. Zinc Reacts With Hydrochloric Acid Formula.
From www.solutionspile.com
[Solved] Zinc reacts with hydrochloric acid according to Zinc Reacts With Hydrochloric Acid Formula Let’s examine the example of the interaction between zinc and hydrochloric acid. in this video, we'll be exploring the reaction between zinc metal and hydrochloric acid. Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g. for example, zinc metal reacts with hydrochloric acid, producing. Zinc Reacts With Hydrochloric Acid Formula.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Zinc Reacts With Hydrochloric Acid Formula zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Let’s examine the example of the interaction between zinc and hydrochloric acid. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g. Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. . Zinc Reacts With Hydrochloric Acid Formula.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Reacts With Hydrochloric Acid Formula for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g. zinc metal reacts with hydrochloric acid according to the balanced equation: in this video, we'll be exploring the reaction between zinc metal and hydrochloric acid. this chemistry video tutorial explains how to. Zinc Reacts With Hydrochloric Acid Formula.
From www.chegg.com
Solved Zinc metal reacts with hydrochloric acid (HCl) Zinc Reacts With Hydrochloric Acid Formula in this video, we'll be exploring the reaction between zinc metal and hydrochloric acid. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. reaction of zinc and hydrochloric acid. zn + hcl =. Zinc Reacts With Hydrochloric Acid Formula.
From shotprofessional22.gitlab.io
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas Zinc Reacts With Hydrochloric Acid Formula zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. zinc metal reacts with hydrochloric acid according to the balanced equation: this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2(. Zinc Reacts With Hydrochloric Acid Formula.
From exoxsryij.blob.core.windows.net
Zinc And Hydrochloric Acid React To Make Zinc Chloride And Hydrogen at Zinc Reacts With Hydrochloric Acid Formula zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Let’s examine the example of the interaction between zinc and hydrochloric acid. zinc metal reacts with hydrochloric acid according to the balanced equation: reaction of zinc and hydrochloric acid. in this video, we'll. Zinc Reacts With Hydrochloric Acid Formula.
From www.nagwa.com
Question Video Describing the Correct Symbol Equation for the Reaction Zinc Reacts With Hydrochloric Acid Formula zinc metal reacts with hydrochloric acid according to the balanced equation: Let’s examine the example of the interaction between zinc and hydrochloric acid. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. zn +. Zinc Reacts With Hydrochloric Acid Formula.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc Reacts With Hydrochloric Acid Formula Let’s examine the example of the interaction between zinc and hydrochloric acid. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g. in this video, we'll be exploring the reaction between zinc metal and hydrochloric acid. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. zinc metal reacts with. Zinc Reacts With Hydrochloric Acid Formula.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0662 Zinc Reacts With Hydrochloric Acid Formula Let’s examine the example of the interaction between zinc and hydrochloric acid. reaction of zinc and hydrochloric acid. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g. zinc metal reacts with hydrochloric acid according to the balanced equation: zinc metal reacts with hydrochloric acid according to the balanced equation: zn + hcl = zncl2 +. Zinc Reacts With Hydrochloric Acid Formula.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc Reacts With Hydrochloric Acid Formula zinc metal reacts with hydrochloric acid according to the balanced equation: for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. reaction of zinc and hydrochloric acid. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. zn + hcl = zncl2 + h2. Zinc Reacts With Hydrochloric Acid Formula.
From www.numerade.com
SOLVED Zinc reacts with hydrochloric acid according to the reaction Zinc Reacts With Hydrochloric Acid Formula Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. reaction of zinc and hydrochloric acid. Let’s examine the example of the interaction between zinc and hydrochloric acid. in this video, we'll be exploring the reaction between zinc metal and hydrochloric acid. zn + hcl = zncl2 + h2 is a single displacement (substitution). Zinc Reacts With Hydrochloric Acid Formula.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc Reacts With Hydrochloric Acid Formula Let’s examine the example of the interaction between zinc and hydrochloric acid. Zn(s) + 2 hcl(aq) → zncl 2 (aq) + h 2 (g) when. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. reaction of zinc and hydrochloric acid. for example, zinc. Zinc Reacts With Hydrochloric Acid Formula.
From www.numerade.com
SOLVED The equation below shows the reaction of zinc with hydrochloric Zinc Reacts With Hydrochloric Acid Formula zinc metal reacts with hydrochloric acid according to the balanced equation: zinc metal reacts with hydrochloric acid according to the balanced equation: zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g. Let’s examine. Zinc Reacts With Hydrochloric Acid Formula.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc Reacts With Hydrochloric Acid Formula for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Zn(s) + 2. Zinc Reacts With Hydrochloric Acid Formula.