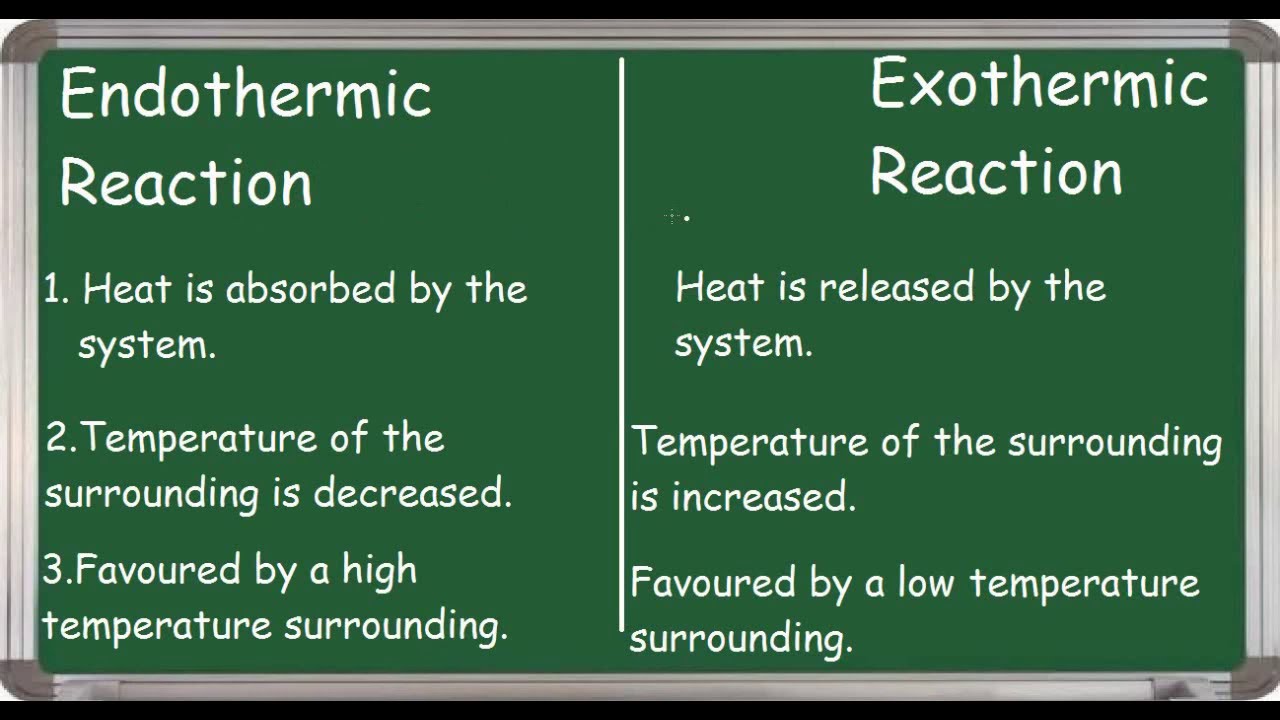
Endothermic Reaction Solution Temperature . Heat is released in a combustion. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. The reaction will proceed towards the liquid phase. There is usually a temperature change. For example, when a bonfire burns it transfers heat energy to the. \text{mol}\) of calcium carbonate decomposes into \(1 \: Dissolution of ammonium nitrate in water, as in. Heat is on the reactant side of the equation. \text{mol}\) of calcium oxide and \(1 \:. When a chemical reaction occurs, energy is transferred to or from the surroundings. When energy is absorbed in an endothermic reaction, the temperature decreases. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g.
from klajlbzfp.blob.core.windows.net
The reaction will proceed towards the liquid phase. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. \text{mol}\) of calcium oxide and \(1 \:. Heat is released in a combustion. When a chemical reaction occurs, energy is transferred to or from the surroundings. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. Heat is on the reactant side of the equation. When energy is absorbed in an endothermic reaction, the temperature decreases. \text{mol}\) of calcium carbonate decomposes into \(1 \: Dissolution of ammonium nitrate in water, as in.
Endothermic Solution Examples at Jeffrey Pleasants blog
Endothermic Reaction Solution Temperature There is usually a temperature change. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. \text{mol}\) of calcium oxide and \(1 \:. There is usually a temperature change. The reaction will proceed towards the liquid phase. \text{mol}\) of calcium carbonate decomposes into \(1 \: Heat is released in a combustion. For example, when a bonfire burns it transfers heat energy to the. When energy is absorbed in an endothermic reaction, the temperature decreases. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. When a chemical reaction occurs, energy is transferred to or from the surroundings. Heat is on the reactant side of the equation. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Dissolution of ammonium nitrate in water, as in.
From slideplayer.com
Chemistry Notes Chapter 2 ppt download Endothermic Reaction Solution Temperature When energy is absorbed in an endothermic reaction, the temperature decreases. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Dissolution of ammonium nitrate in water, as in. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases.. Endothermic Reaction Solution Temperature.
From www.numerade.com
SOLVED Which one of the following given graphs represents the Endothermic Reaction Solution Temperature The reaction will proceed towards the liquid phase. \text{mol}\) of calcium oxide and \(1 \:. Heat is on the reactant side of the equation. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. \text{mol}\) of calcium carbonate decomposes into \(1 \: When a chemical reaction occurs, energy is transferred to or from the surroundings. Heat. Endothermic Reaction Solution Temperature.
From studymind.co.uk
Endothermic vs Exothermic Reactions (GCSE Chemistry) Study Mind Endothermic Reaction Solution Temperature By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Heat is on the reactant side of the equation. \text{mol}\) of calcium oxide and \(1 \:. \text{mol}\) of calcium carbonate decomposes into \(1 \: When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. The reaction will proceed towards the liquid phase. Exothermic. Endothermic Reaction Solution Temperature.
From www.bbc.co.uk
What are exothermic and endothermic reactions? BBC Bitesize Endothermic Reaction Solution Temperature By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. The reaction will proceed towards the liquid phase. Dissolution of ammonium nitrate in water, as in. Heat is on the reactant side of the equation. Heat is released in a combustion. \text{mol}\) of calcium oxide and \(1 \:. When energy is released in an exothermic reaction, the temperature. Endothermic Reaction Solution Temperature.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction Solution Temperature Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Heat is on the reactant side of the equation. When a chemical reaction occurs, energy is transferred to or from the surroundings. For example, when a bonfire burns it transfers heat energy to the.. Endothermic Reaction Solution Temperature.
From improove.in
Experiment 16 Heat generation during reaction Improove Endothermic Reaction Solution Temperature For example, when a bonfire burns it transfers heat energy to the. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. Heat is released in a combustion. When energy is absorbed in an endothermic reaction, the temperature decreases. When a chemical reaction occurs, energy is transferred to or from the surroundings. There is usually. Endothermic Reaction Solution Temperature.
From sciencenotes.org
Exothermic Reactions Definition and Examples Endothermic Reaction Solution Temperature Heat is released in a combustion. For example, when a bonfire burns it transfers heat energy to the. \text{mol}\) of calcium oxide and \(1 \:. The reaction will proceed towards the liquid phase. \text{mol}\) of calcium carbonate decomposes into \(1 \: There is usually a temperature change. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings. Endothermic Reaction Solution Temperature.
From slideplayer.com
12.1 Chemical reactions that involve heat ppt download Endothermic Reaction Solution Temperature \text{mol}\) of calcium carbonate decomposes into \(1 \: When energy is absorbed in an endothermic reaction, the temperature decreases. Heat is released in a combustion. Dissolution of ammonium nitrate in water, as in. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. \text{mol}\). Endothermic Reaction Solution Temperature.
From mmerevise.co.uk
Enthalpy Changes and Calorimetry MME Endothermic Reaction Solution Temperature The reaction will proceed towards the liquid phase. Dissolution of ammonium nitrate in water, as in. When a chemical reaction occurs, energy is transferred to or from the surroundings. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. Heat is on the reactant side of the equation. By definition, an exothermic reaction gives off. Endothermic Reaction Solution Temperature.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Solution Temperature For example, when a bonfire burns it transfers heat energy to the. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. When energy is absorbed in an endothermic reaction, the temperature decreases. Heat is released in a combustion. \text{mol}\) of calcium carbonate decomposes into \(1 \: Dissolution of ammonium nitrate in water, as in. By. Endothermic Reaction Solution Temperature.
From letstalkscience.ca
The Cold Pack A Chilly Example of an Endothermic Reaction Let's Talk Endothermic Reaction Solution Temperature Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. When a chemical reaction occurs, energy is transferred to or from the surroundings. Heat is released in a combustion. When energy is absorbed in an endothermic reaction, the temperature decreases. Heat is on the. Endothermic Reaction Solution Temperature.
From signalticket9.pythonanywhere.com
Simple Endothermic Reactions With Equations Reaction Balanced Equation Endothermic Reaction Solution Temperature Heat is on the reactant side of the equation. For example, when a bonfire burns it transfers heat energy to the. When energy is absorbed in an endothermic reaction, the temperature decreases. \text{mol}\) of calcium oxide and \(1 \:. When a chemical reaction occurs, energy is transferred to or from the surroundings. There is usually a temperature change. The reaction. Endothermic Reaction Solution Temperature.
From askfilo.com
The change in temperature noticed i. endothermic reaction ii. the reactio.. Endothermic Reaction Solution Temperature When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. \text{mol}\) of calcium carbonate decomposes into \(1 \: There is usually a temperature change. Dissolution of ammonium nitrate in water, as in. When energy is absorbed in an endothermic reaction, the temperature decreases. Heat is released in a combustion. For example, when a bonfire burns. Endothermic Reaction Solution Temperature.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Solution Temperature \text{mol}\) of calcium carbonate decomposes into \(1 \: For example, when a bonfire burns it transfers heat energy to the. The reaction will proceed towards the liquid phase. There is usually a temperature change. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. When a chemical reaction occurs, energy is transferred to or from. Endothermic Reaction Solution Temperature.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction Solution Temperature Heat is released in a combustion. When energy is absorbed in an endothermic reaction, the temperature decreases. There is usually a temperature change. \text{mol}\) of calcium carbonate decomposes into \(1 \: The reaction will proceed towards the liquid phase. Dissolution of ammonium nitrate in water, as in. \text{mol}\) of calcium oxide and \(1 \:. When a chemical reaction occurs, energy. Endothermic Reaction Solution Temperature.
From slideplayer.com
Chemical Changes Physical changes (Ch. 5) involve only changes in the Endothermic Reaction Solution Temperature When energy is absorbed in an endothermic reaction, the temperature decreases. Heat is released in a combustion. \text{mol}\) of calcium carbonate decomposes into \(1 \: By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. For example, when a bonfire burns it transfers heat energy to the. When a chemical reaction occurs, energy is transferred to or from. Endothermic Reaction Solution Temperature.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Solution Temperature \text{mol}\) of calcium oxide and \(1 \:. Dissolution of ammonium nitrate in water, as in. Heat is on the reactant side of the equation. When a chemical reaction occurs, energy is transferred to or from the surroundings. For example, when a bonfire burns it transfers heat energy to the. Heat is released in a combustion. There is usually a temperature. Endothermic Reaction Solution Temperature.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Solution Temperature \text{mol}\) of calcium carbonate decomposes into \(1 \: Heat is released in a combustion. Dissolution of ammonium nitrate in water, as in. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. When a chemical reaction occurs, energy is transferred to or from. Endothermic Reaction Solution Temperature.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Solution Temperature When a chemical reaction occurs, energy is transferred to or from the surroundings. For example, when a bonfire burns it transfers heat energy to the. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. The reaction will proceed towards the liquid phase. \text{mol}\) of calcium carbonate decomposes into \(1 \: Heat is released in a combustion. Exothermic. Endothermic Reaction Solution Temperature.
From trailerspotting.blogspot.com
40 enthalpy level diagram Endothermic Reaction Solution Temperature By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. Dissolution of ammonium nitrate in water, as in. \text{mol}\) of calcium carbonate decomposes into \(1 \: For example, when a bonfire burns it transfers heat energy to the. Heat is on the reactant. Endothermic Reaction Solution Temperature.
From chart-studio.plotly.com
Endothermic and Exothermic Reactions Graph scatter chart made by Endothermic Reaction Solution Temperature There is usually a temperature change. For example, when a bonfire burns it transfers heat energy to the. \text{mol}\) of calcium carbonate decomposes into \(1 \: When a chemical reaction occurs, energy is transferred to or from the surroundings. Heat is released in a combustion. When energy is absorbed in an endothermic reaction, the temperature decreases. Heat is on the. Endothermic Reaction Solution Temperature.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Solution Temperature When a chemical reaction occurs, energy is transferred to or from the surroundings. The reaction will proceed towards the liquid phase. Heat is released in a combustion. \text{mol}\) of calcium oxide and \(1 \:. There is usually a temperature change. For example, when a bonfire burns it transfers heat energy to the. When energy is absorbed in an endothermic reaction,. Endothermic Reaction Solution Temperature.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Endothermic Reaction Solution Temperature When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. For example, when a bonfire burns it transfers heat energy to the. \text{mol}\) of calcium oxide and \(1 \:. Heat is on the reactant side of the equation. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. When a chemical. Endothermic Reaction Solution Temperature.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Solution Temperature Heat is released in a combustion. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Heat is on the reactant side of the equation. There is usually a temperature change. For example, when a bonfire burns it transfers heat energy to the. When energy is absorbed in an endothermic reaction, the temperature decreases. \text{mol}\) of calcium carbonate. Endothermic Reaction Solution Temperature.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Endothermic Reaction Solution Temperature For example, when a bonfire burns it transfers heat energy to the. Heat is on the reactant side of the equation. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. When a chemical reaction occurs, energy is transferred to or from the surroundings. \text{mol}\) of calcium carbonate decomposes into \(1 \: When energy is. Endothermic Reaction Solution Temperature.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Solution Temperature When a chemical reaction occurs, energy is transferred to or from the surroundings. Dissolution of ammonium nitrate in water, as in. \text{mol}\) of calcium oxide and \(1 \:. \text{mol}\) of calcium carbonate decomposes into \(1 \: When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. Heat is on the reactant side of the equation.. Endothermic Reaction Solution Temperature.
From pharmaguides.in
6.5 Why Endothermic Reaction Required Energy Endothermic Reaction Solution Temperature Dissolution of ammonium nitrate in water, as in. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. For example, when a bonfire burns it transfers heat energy to the. \text{mol}\) of calcium oxide and \(1 \:. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. Heat is on the. Endothermic Reaction Solution Temperature.
From www.savemyexams.com
Endothermic & Exothermic Reactions Cambridge O Level Chemistry Endothermic Reaction Solution Temperature Dissolution of ammonium nitrate in water, as in. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. When a chemical reaction occurs, energy is transferred to or from the surroundings. \text{mol}\) of calcium oxide and \(1 \:. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. Heat is on. Endothermic Reaction Solution Temperature.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Solution Temperature When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. \text{mol}\) of calcium oxide and \(1 \:. Dissolution of ammonium nitrate in water, as in. Heat is on the reactant side of the equation. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Heat is released in a combustion. There is usually. Endothermic Reaction Solution Temperature.
From mungfali.com
Exothermic Reaction Energy Profile Diagram Endothermic Reaction Solution Temperature For example, when a bonfire burns it transfers heat energy to the. The reaction will proceed towards the liquid phase. When energy is absorbed in an endothermic reaction, the temperature decreases. Dissolution of ammonium nitrate in water, as in. Heat is released in a combustion. \text{mol}\) of calcium carbonate decomposes into \(1 \: When energy is released in an exothermic. Endothermic Reaction Solution Temperature.
From slideplayer.com
Learning Objective Describe energy transfers during a reaction ppt Endothermic Reaction Solution Temperature The reaction will proceed towards the liquid phase. When a chemical reaction occurs, energy is transferred to or from the surroundings. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Heat is released in a combustion. For example, when a bonfire burns it transfers heat energy to the. When energy is absorbed in an endothermic reaction, the. Endothermic Reaction Solution Temperature.
From www.pinterest.com
Endothermic and Exothermic Reactions Lab ⋆ Exothermic Endothermic Reaction Solution Temperature Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Heat is on the reactant side of the equation. Heat is released in a combustion. The reaction will proceed towards the liquid phase. When a chemical reaction occurs, energy is transferred to or from. Endothermic Reaction Solution Temperature.
From slideplayer.com
Chemistry Notes Chapter 2 ppt download Endothermic Reaction Solution Temperature Heat is on the reactant side of the equation. \text{mol}\) of calcium oxide and \(1 \:. Heat is released in a combustion. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Dissolution of ammonium nitrate in water, as in. When a chemical. Endothermic Reaction Solution Temperature.
From klajlbzfp.blob.core.windows.net
Endothermic Solution Examples at Jeffrey Pleasants blog Endothermic Reaction Solution Temperature \text{mol}\) of calcium carbonate decomposes into \(1 \: For example, when a bonfire burns it transfers heat energy to the. Heat is released in a combustion. \text{mol}\) of calcium oxide and \(1 \:. When energy is absorbed in an endothermic reaction, the temperature decreases. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. There. Endothermic Reaction Solution Temperature.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Endothermic Reaction Solution Temperature When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. Heat is on the reactant side of the equation. Dissolution of ammonium nitrate in water, as in. Heat is released in a combustion. When a chemical reaction occurs, energy is transferred to or from the surroundings. By definition, an exothermic reaction gives off heat, and. Endothermic Reaction Solution Temperature.