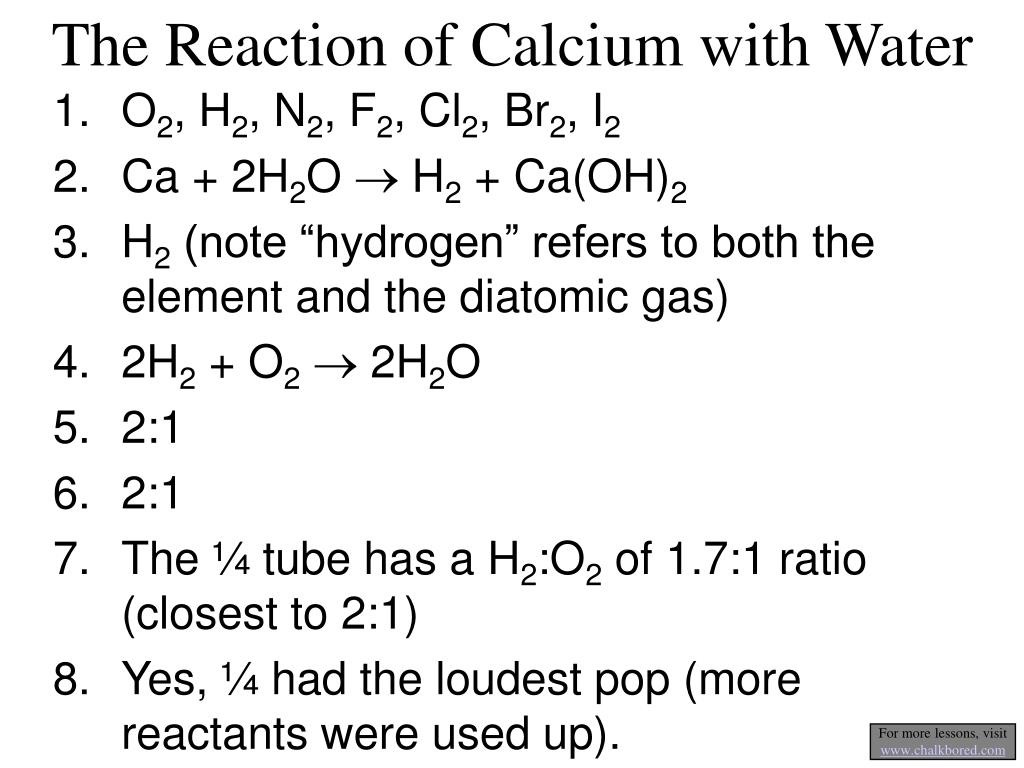
Is Calcium Reactive With Water . 9 rows the reactivity series ranks metals by how readily they react. Part of ncssm core collection: The hydroxides of calcium, strontium, and barium are only slightly soluble in water; These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. However, enough hydroxide ions are produced to make a basic environment. Calcium + water calcium hydroxide + hydrogen. When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with the liberation of hydrogen gas h 2. Calcium reacts readily with water. These metals react vigorously with water, although not as violently as. The general reaction of calcium,. More reactive metals displace less reactive metals from their compounds and. Bubbles of hydrogen gas are given off, and a white precipitate. Strontium and barium have reactivities similar to that of. Calcium, for example, reacts fairly vigorously with cold water in an exothermic reaction. This video shows the physical properties of ca metal and its reaction.
from www.slideserve.com
However, enough hydroxide ions are produced to make a basic environment. Calcium, for example, reacts fairly vigorously with cold water in an exothermic reaction. This video shows the physical properties of ca metal and its reaction. 9 rows the reactivity series ranks metals by how readily they react. Strontium and barium have reactivities similar to that of. The hydroxides of calcium, strontium, and barium are only slightly soluble in water; When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with the liberation of hydrogen gas h 2. The general reaction of calcium,. Part of ncssm core collection: More reactive metals displace less reactive metals from their compounds and.
PPT The Reaction of Calcium with Water PowerPoint Presentation, free
Is Calcium Reactive With Water This video shows the physical properties of ca metal and its reaction. These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. However, enough hydroxide ions are produced to make a basic environment. The hydroxides of calcium, strontium, and barium are only slightly soluble in water; Part of ncssm core collection: Calcium, for example, reacts fairly vigorously with cold water in an exothermic reaction. Bubbles of hydrogen gas are given off, and a white precipitate. Strontium and barium have reactivities similar to that of. Calcium + water calcium hydroxide + hydrogen. More reactive metals displace less reactive metals from their compounds and. This video shows the physical properties of ca metal and its reaction. When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with the liberation of hydrogen gas h 2. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts to become milky as solid calcium hydroxide. 9 rows the reactivity series ranks metals by how readily they react. These metals react vigorously with water, although not as violently as. The general reaction of calcium,.
From www.mdpi.com
Antioxidants Free FullText Calcium and Reactive Oxygen Species Is Calcium Reactive With Water More reactive metals displace less reactive metals from their compounds and. When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with the liberation of hydrogen gas h 2. Calcium reacts readily with water. Part of ncssm core collection: These metals react vigorously with water, although not as violently as. However, enough hydroxide. Is Calcium Reactive With Water.
From www.hanlin.com
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:2.4.1 Metals Reacting with Is Calcium Reactive With Water These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. This video shows the physical properties of ca metal and its reaction. Bubbles of hydrogen gas are given off, and a white precipitate. Calcium reacts readily with water. Calcium + water calcium hydroxide + hydrogen. 9 rows the reactivity series ranks metals by how. Is Calcium Reactive With Water.
From fphoto.photoshelter.com
science chemistry reaction exothermic calcium water Fundamental Is Calcium Reactive With Water More reactive metals displace less reactive metals from their compounds and. However, enough hydroxide ions are produced to make a basic environment. Calcium reacts readily with water. Strontium and barium have reactivities similar to that of. Calcium, for example, reacts fairly vigorously with cold water in an exothermic reaction. Bubbles of hydrogen gas are given off, and a white precipitate.. Is Calcium Reactive With Water.
From www.sciencephoto.com
Calcium Reacts with Water, 2 of 4 Stock Image C030/7953 Science Is Calcium Reactive With Water More reactive metals displace less reactive metals from their compounds and. Part of ncssm core collection: Strontium and barium have reactivities similar to that of. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts to become milky as solid calcium hydroxide. Bubbles of hydrogen gas are given off, and a white. Is Calcium Reactive With Water.
From proper-cooking.info
Periodic Table Trends Reactivity Is Calcium Reactive With Water The general reaction of calcium,. The hydroxides of calcium, strontium, and barium are only slightly soluble in water; Part of ncssm core collection: When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with the liberation of hydrogen gas h 2. Calcium, for example, reacts fairly vigorously with cold water in an exothermic. Is Calcium Reactive With Water.
From pixels.com
Calcium Reacting With Water Photograph by Andrew Lambert Photography Is Calcium Reactive With Water Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts to become milky as solid calcium hydroxide. Bubbles of hydrogen gas are given off, and a white precipitate. When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with the liberation of hydrogen gas h. Is Calcium Reactive With Water.
From www.slideserve.com
PPT Magnesium reacts quicker than copper and iron. There is a league Is Calcium Reactive With Water The hydroxides of calcium, strontium, and barium are only slightly soluble in water; Calcium, for example, reacts fairly vigorously with cold water in an exothermic reaction. Calcium reacts readily with water. However, enough hydroxide ions are produced to make a basic environment. When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with. Is Calcium Reactive With Water.
From www.tes.com
Metals and Acids Reactivity Series KS4 Edexcel 91 Teaching Resources Is Calcium Reactive With Water Calcium, for example, reacts fairly vigorously with cold water in an exothermic reaction. The hydroxides of calcium, strontium, and barium are only slightly soluble in water; The general reaction of calcium,. However, enough hydroxide ions are produced to make a basic environment. These metals react vigorously with water, although not as violently as. When calcium ca reacts with water h. Is Calcium Reactive With Water.
From www.youtube.com
Calcium in Water YouTube Is Calcium Reactive With Water The hydroxides of calcium, strontium, and barium are only slightly soluble in water; More reactive metals displace less reactive metals from their compounds and. Part of ncssm core collection: These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. These metals react vigorously with water, although not as violently as. 9 rows the reactivity. Is Calcium Reactive With Water.
From byjus.com
Which metal reacts with cold water? Is Calcium Reactive With Water Calcium, for example, reacts fairly vigorously with cold water in an exothermic reaction. These metals react vigorously with water, although not as violently as. The hydroxides of calcium, strontium, and barium are only slightly soluble in water; However, enough hydroxide ions are produced to make a basic environment. When calcium ca reacts with water h 2 o, calcium hydroxide ca. Is Calcium Reactive With Water.
From www.alamy.com
reactivity of different metals with hydrochloric acid calcium Stock Is Calcium Reactive With Water This video shows the physical properties of ca metal and its reaction. These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. Strontium and barium have reactivities similar to that of. Calcium + water calcium hydroxide + hydrogen. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) ,. Is Calcium Reactive With Water.
From www.slideserve.com
PPT The Reaction of Calcium with Water PowerPoint Presentation, free Is Calcium Reactive With Water These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. More reactive metals displace less reactive metals from their compounds and. Calcium, for example, reacts fairly vigorously with cold water in an exothermic reaction. Calcium reacts readily with water. These metals react vigorously with water, although not as violently as. However, enough hydroxide ions. Is Calcium Reactive With Water.
From raphael-has-chung.blogspot.com
Why Is Calcium More Reactive Than Magnesium RaphaelhasChung Is Calcium Reactive With Water This video shows the physical properties of ca metal and its reaction. Calcium, for example, reacts fairly vigorously with cold water in an exothermic reaction. The hydroxides of calcium, strontium, and barium are only slightly soluble in water; However, enough hydroxide ions are produced to make a basic environment. Strontium and barium have reactivities similar to that of. These metals. Is Calcium Reactive With Water.
From classfullcasandra.z21.web.core.windows.net
Reactivity Chart Periodic Table Is Calcium Reactive With Water Calcium reacts readily with water. Strontium and barium have reactivities similar to that of. However, enough hydroxide ions are produced to make a basic environment. Part of ncssm core collection: Bubbles of hydrogen gas are given off, and a white precipitate. When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with the. Is Calcium Reactive With Water.
From www.tutormyself.com
215 understand how metals can be arranged in a reactivity series based Is Calcium Reactive With Water Calcium reacts readily with water. Strontium and barium have reactivities similar to that of. More reactive metals displace less reactive metals from their compounds and. These metals react vigorously with water, although not as violently as. The hydroxides of calcium, strontium, and barium are only slightly soluble in water; This video shows the physical properties of ca metal and its. Is Calcium Reactive With Water.
From mammothmemory.net
Calcium in group 2 is less reactive but still pungent Is Calcium Reactive With Water This video shows the physical properties of ca metal and its reaction. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts to become milky as solid calcium hydroxide. Part of ncssm core collection: The general reaction of calcium,. However, enough hydroxide ions are produced to make a basic environment. More reactive. Is Calcium Reactive With Water.
From woelen.homescience.net
Science made alive Chemistry/Experiments Is Calcium Reactive With Water Part of ncssm core collection: More reactive metals displace less reactive metals from their compounds and. When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with the liberation of hydrogen gas h 2. Calcium reacts readily with water. These metals react vigorously with water, although not as violently as. Calcium, for example,. Is Calcium Reactive With Water.
From zabir.ru
Water reaction Is Calcium Reactive With Water 9 rows the reactivity series ranks metals by how readily they react. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts to become milky as solid calcium hydroxide. Calcium reacts readily with water. This video shows the physical properties of ca metal and its reaction. Calcium, for example, reacts fairly vigorously. Is Calcium Reactive With Water.
From fphoto.photoshelter.com
science chemistry reaction exothermic calcium water Fundamental Is Calcium Reactive With Water Calcium, for example, reacts fairly vigorously with cold water in an exothermic reaction. These metals react vigorously with water, although not as violently as. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts to become milky as solid calcium hydroxide. The hydroxides of calcium, strontium, and barium are only slightly soluble. Is Calcium Reactive With Water.
From fphoto.photoshelter.com
science chemistry reaction exothermic calcium water Fundamental Is Calcium Reactive With Water These metals react vigorously with water, although not as violently as. Calcium, for example, reacts fairly vigorously with cold water in an exothermic reaction. This video shows the physical properties of ca metal and its reaction. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts to become milky as solid calcium. Is Calcium Reactive With Water.
From www.alamy.com
Calcium reacting with water Stock Photo Alamy Is Calcium Reactive With Water The hydroxides of calcium, strontium, and barium are only slightly soluble in water; More reactive metals displace less reactive metals from their compounds and. However, enough hydroxide ions are produced to make a basic environment. Bubbles of hydrogen gas are given off, and a white precipitate. Part of ncssm core collection: Ca (s) + 2h 2 o (l) ca (oh). Is Calcium Reactive With Water.
From constructionhow.com
Determination of CalciumMagnesium Hardness of Water Construction How Is Calcium Reactive With Water Part of ncssm core collection: Calcium + water calcium hydroxide + hydrogen. Strontium and barium have reactivities similar to that of. These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. Bubbles of hydrogen gas are given off, and a white precipitate. The hydroxides of calcium, strontium, and barium are only slightly soluble in. Is Calcium Reactive With Water.
From pixels.com
Calcium Reacting With Water Photograph by Science Photo Library Pixels Is Calcium Reactive With Water When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with the liberation of hydrogen gas h 2. These metals react vigorously with water, although not as violently as. Strontium and barium have reactivities similar to that of. However, enough hydroxide ions are produced to make a basic environment. The general reaction of. Is Calcium Reactive With Water.
From www.chemistrylearner.com
Reactivity Series Definition and Chart Is Calcium Reactive With Water Calcium, for example, reacts fairly vigorously with cold water in an exothermic reaction. Calcium + water calcium hydroxide + hydrogen. These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. These metals react vigorously with water, although not as violently as. More reactive metals displace less reactive metals from their compounds and. The general. Is Calcium Reactive With Water.
From www.youtube.com
Reaction of Magnesium and Water (Mg + H2O) YouTube Is Calcium Reactive With Water Calcium + water calcium hydroxide + hydrogen. Calcium, for example, reacts fairly vigorously with cold water in an exothermic reaction. Strontium and barium have reactivities similar to that of. However, enough hydroxide ions are produced to make a basic environment. Part of ncssm core collection: When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is. Is Calcium Reactive With Water.
From www.thesciencehive.co.uk
The Reactivity Series (GCSE) — the science hive Is Calcium Reactive With Water The hydroxides of calcium, strontium, and barium are only slightly soluble in water; Part of ncssm core collection: Strontium and barium have reactivities similar to that of. Calcium reacts readily with water. When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with the liberation of hydrogen gas h 2. These metals react. Is Calcium Reactive With Water.
From quizdbcornwallis.z21.web.core.windows.net
What Makes An Element More Reactive Is Calcium Reactive With Water Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts to become milky as solid calcium hydroxide. The hydroxides of calcium, strontium, and barium are only slightly soluble in water; Calcium, for example, reacts fairly vigorously with cold water in an exothermic reaction. Calcium + water calcium hydroxide + hydrogen. These metals. Is Calcium Reactive With Water.
From getrevising.co.uk
The Reactivity Series Revision Notes in GCSE Chemistry Is Calcium Reactive With Water Part of ncssm core collection: Calcium reacts readily with water. Calcium, for example, reacts fairly vigorously with cold water in an exothermic reaction. When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with the liberation of hydrogen gas h 2. This video shows the physical properties of ca metal and its reaction.. Is Calcium Reactive With Water.
From www.chegg.com
Consider the reaction of Calcium carbide and water as Is Calcium Reactive With Water The general reaction of calcium,. Calcium reacts readily with water. More reactive metals displace less reactive metals from their compounds and. When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with the liberation of hydrogen gas h 2. Strontium and barium have reactivities similar to that of. Bubbles of hydrogen gas are. Is Calcium Reactive With Water.
From www.mammothmemory.net
Calcium is denser than group 1 metals so sinks and reacts Is Calcium Reactive With Water However, enough hydroxide ions are produced to make a basic environment. More reactive metals displace less reactive metals from their compounds and. When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with the liberation of hydrogen gas h 2. The general reaction of calcium,. Calcium, for example, reacts fairly vigorously with cold. Is Calcium Reactive With Water.
From www.sciencephoto.com
Calcium Reacting with Water Stock Image C027/9558 Science Photo Is Calcium Reactive With Water When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with the liberation of hydrogen gas h 2. 9 rows the reactivity series ranks metals by how readily they react. The hydroxides of calcium, strontium, and barium are only slightly soluble in water; Calcium reacts readily with water. Part of ncssm core collection:. Is Calcium Reactive With Water.
From utedzz.blogspot.com
Periodic Table Reactivity Series Of Elements Periodic Table Timeline Is Calcium Reactive With Water These metals react vigorously with water, although not as violently as. However, enough hydroxide ions are produced to make a basic environment. 9 rows the reactivity series ranks metals by how readily they react. When calcium ca reacts with water h 2 o, calcium hydroxide ca (oh) 2 is formed along with the liberation of hydrogen gas h 2. More. Is Calcium Reactive With Water.
From byjus.com
How Calcium or Magnesium can displace Sodium or Potassium from Is Calcium Reactive With Water More reactive metals displace less reactive metals from their compounds and. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts to become milky as solid calcium hydroxide. Calcium reacts readily with water. However, enough hydroxide ions are produced to make a basic environment. Strontium and barium have reactivities similar to that. Is Calcium Reactive With Water.
From www.pinterest.com
Calcium, Reactive Oxygen Species, and Synaptic Plasticity Reactive Is Calcium Reactive With Water This video shows the physical properties of ca metal and its reaction. 9 rows the reactivity series ranks metals by how readily they react. Calcium + water calcium hydroxide + hydrogen. More reactive metals displace less reactive metals from their compounds and. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) + h 2 (g) , it starts. Is Calcium Reactive With Water.
From ar.inspiredpencil.com
Chemical Reactivity Matrix Is Calcium Reactive With Water These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. The general reaction of calcium,. Calcium reacts readily with water. More reactive metals displace less reactive metals from their compounds and. These metals react vigorously with water, although not as violently as. Ca (s) + 2h 2 o (l) ca (oh) 2 (aq) +. Is Calcium Reactive With Water.