Bomb Calorimetry Worksheet . 1) a compound is burned in a bomb calorimeter that. Cp (h2o) = 4.184 j / g0c h = mcp t. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. The experimental data ignores the mass and. explain why this is, based on your knowledge of how bomb calorimetry works. 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. one method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator.
from www.chegg.com
explain why this is, based on your knowledge of how bomb calorimetry works. one method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. 1) a compound is burned in a bomb calorimeter that. Cp (h2o) = 4.184 j / g0c h = mcp t. The experimental data ignores the mass and.
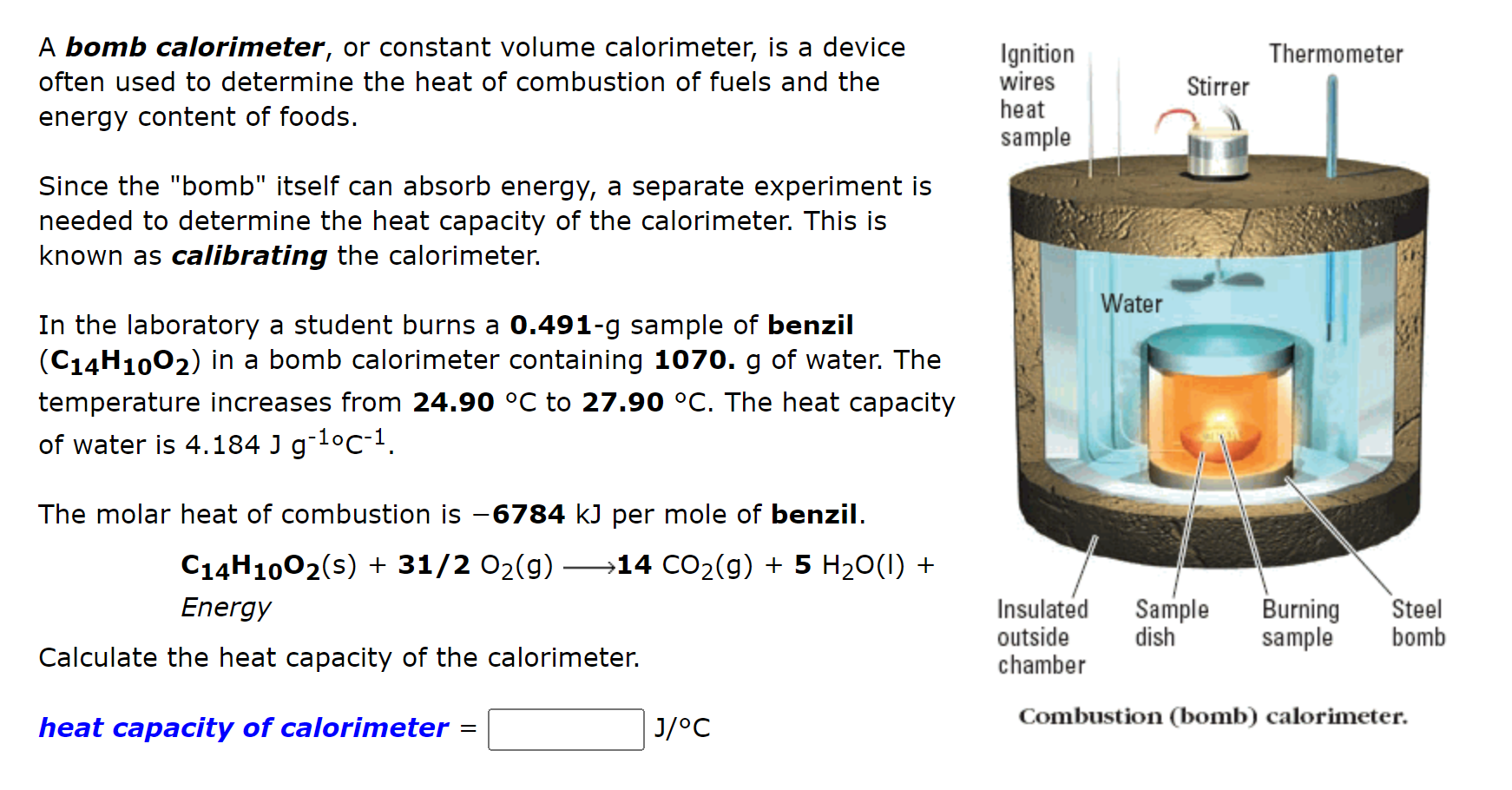
Solved Thermometer A bomb calorimeter, or constant volume
Bomb Calorimetry Worksheet explain why this is, based on your knowledge of how bomb calorimetry works. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. The experimental data ignores the mass and. explain why this is, based on your knowledge of how bomb calorimetry works. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. 1) a compound is burned in a bomb calorimeter that. 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing. one method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator. Cp (h2o) = 4.184 j / g0c h = mcp t.
From www.scribd.com
Questions Calorimetry Using A Bomb Calorimeter PDF Power Station Bomb Calorimetry Worksheet 1) a compound is burned in a bomb calorimeter that. one method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator. Cp (h2o) = 4.184 j / g0c h = mcp t. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb. Bomb Calorimetry Worksheet.
From www.studocu.com
Experiment 4 Bomb Calorimetry W2021 Outline 01 Compleated Chemistry Bomb Calorimetry Worksheet one method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing. Compound a is. Bomb Calorimetry Worksheet.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimetry Worksheet a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. explain why this is, based on your knowledge of how bomb calorimetry works. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. Cp (h2o) =. Bomb Calorimetry Worksheet.
From www.studypool.com
SOLUTION Bomb calorimeter study material Studypool Bomb Calorimetry Worksheet a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. 1) a compound is burned in a bomb calorimeter that. Cp (h2o) = 4.184 j / g0c h = mcp t. one method of generating electricity is by burning coal to heat water, which produces steam that. Bomb Calorimetry Worksheet.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimetry Worksheet one method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator. 1) a compound is burned in a bomb calorimeter that. Cp (h2o) = 4.184 j / g0c h = mcp t. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid. Bomb Calorimetry Worksheet.
From fyozfywtx.blob.core.windows.net
Bomb Calorimetry Examples at Alex Kowalski blog Bomb Calorimetry Worksheet Cp (h2o) = 4.184 j / g0c h = mcp t. explain why this is, based on your knowledge of how bomb calorimetry works. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing.. Bomb Calorimetry Worksheet.
From www.chegg.com
Solved BOMB CALORIMETRY LAB WORKSHEETS WORKSHEETS DO NOT Bomb Calorimetry Worksheet explain why this is, based on your knowledge of how bomb calorimetry works. 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing. 1) a compound is burned in a bomb calorimeter that. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. one method of generating electricity is by. Bomb Calorimetry Worksheet.
From exyqlzddm.blob.core.windows.net
Calorimeter Examples Chemistry at Sima Montgomery blog Bomb Calorimetry Worksheet Cp (h2o) = 4.184 j / g0c h = mcp t. explain why this is, based on your knowledge of how bomb calorimetry works. The experimental data ignores the mass and. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. Compound a is burned in a bomb. Bomb Calorimetry Worksheet.
From studylib.net
Bomb Calorimeter Lab Sheet Bomb Calorimetry Worksheet 1) a compound is burned in a bomb calorimeter that. one method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator. 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned. Bomb Calorimetry Worksheet.
From exykexyqb.blob.core.windows.net
Bomb Of Calorimetry at Jeanne Gee blog Bomb Calorimetry Worksheet 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing. one method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. The experimental data ignores the mass and. Cp (h2o) = 4.184. Bomb Calorimetry Worksheet.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimetry Worksheet a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. 1) a compound is burned in a bomb calorimeter that. explain why this is, based on your knowledge of how bomb calorimetry works.. Bomb Calorimetry Worksheet.
From studylib.net
Calorimetry Worksheet Bomb Calorimetry Worksheet Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Cp (h2o) = 4.184 j / g0c h = mcp t. one method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is. Bomb Calorimetry Worksheet.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Bomb Calorimetry Worksheet explain why this is, based on your knowledge of how bomb calorimetry works. Cp (h2o) = 4.184 j / g0c h = mcp t. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. The experimental data ignores the mass and. solving bomb calorimeter problems a. Bomb Calorimetry Worksheet.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimetry Worksheet Cp (h2o) = 4.184 j / g0c h = mcp t. 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. one method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator.. Bomb Calorimetry Worksheet.
From www.studypool.com
SOLUTION Bomb calorimeter with examples instrument calculation and Bomb Calorimetry Worksheet The experimental data ignores the mass and. 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Cp (h2o) = 4.184. Bomb Calorimetry Worksheet.
From www.chegg.com
Solved Thermometer A bomb calorimeter, or constant volume Bomb Calorimetry Worksheet 1) a compound is burned in a bomb calorimeter that. explain why this is, based on your knowledge of how bomb calorimetry works. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. The. Bomb Calorimetry Worksheet.
From www.chegg.com
Solved BOMB CALORIMETRY LAB WORKSHEETS WORKSHEETS DO NOT Bomb Calorimetry Worksheet a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. 1) a compound is burned in a bomb calorimeter that. one method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator. Cp (h2o) = 4.184 j / g0c. Bomb Calorimetry Worksheet.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimetry Worksheet The experimental data ignores the mass and. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. Cp (h2o) = 4.184 j / g0c h = mcp t. 1) a compound is burned in a. Bomb Calorimetry Worksheet.
From studylib.net
Calorimetry practice Bomb Calorimetry Worksheet 1) a compound is burned in a bomb calorimeter that. 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing. Cp (h2o) = 4.184 j / g0c h = mcp t. explain why this is, based on your knowledge of how bomb calorimetry works. solving bomb calorimeter problems a 1.000 g sample of octane. Bomb Calorimetry Worksheet.
From studylib.net
Calorimetry Worksheet Bomb Calorimetry Worksheet Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. 1) a compound is burned in a bomb calorimeter that. Cp (h2o) = 4.184 j / g0c h = mcp t. a bomb calorimeter. Bomb Calorimetry Worksheet.
From narodnatribuna.info
Enthalpy Calorimetry Equation Bomb Calorimetry Worksheet a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. 1) a compound is burned in a bomb calorimeter that. one method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator. solving bomb calorimeter problems a 1.000. Bomb Calorimetry Worksheet.
From printablefullombus.z21.web.core.windows.net
Calorimeter Worksheets Bomb Calorimetry Worksheet solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. The experimental data ignores the mass and. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. Cp (h2o) = 4.184 j / g0c h = mcp. Bomb Calorimetry Worksheet.
From www.studocu.com
Exp1 Bomb+Calorimetry A B Experiment 1 Determination of enthalpy of Bomb Calorimetry Worksheet a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. Cp (h2o) = 4.184 j / g0c h = mcp t. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. The experimental data ignores the mass. Bomb Calorimetry Worksheet.
From studylib.net
Calorimetry Worksheet Bomb Calorimetry Worksheet solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. Cp (h2o) = 4.184 j / g0c h = mcp t. explain why this is, based on your knowledge of how bomb calorimetry works. 1) a compound is burned in a bomb calorimeter that. The experimental data ignores. Bomb Calorimetry Worksheet.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Bomb Calorimetry Worksheet one method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. 1) if i. Bomb Calorimetry Worksheet.
From studylib.net
CalorimetryWorksheetsolutionscopy Bomb Calorimetry Worksheet Cp (h2o) = 4.184 j / g0c h = mcp t. explain why this is, based on your knowledge of how bomb calorimetry works. 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat. Bomb Calorimetry Worksheet.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimetry Worksheet The experimental data ignores the mass and. explain why this is, based on your knowledge of how bomb calorimetry works. Cp (h2o) = 4.184 j / g0c h = mcp t. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. 1) a compound is burned in. Bomb Calorimetry Worksheet.
From www.onlineworksheet.my.id
Calorimetry Worksheet Answer Key Onlineworksheet.my.id Bomb Calorimetry Worksheet Cp (h2o) = 4.184 j / g0c h = mcp t. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. explain why this is, based on your knowledge of how bomb calorimetry. Bomb Calorimetry Worksheet.
From www.chegg.com
Solved BOMB CALORIMETRY LAB WORKSHEETS WORKSHEETS DO NOT Bomb Calorimetry Worksheet a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. explain why this is, based on your knowledge of how bomb calorimetry works. Cp (h2o) = 4.184 j / g0c h = mcp t. 1) a compound is burned in a bomb calorimeter that. 1) if i. Bomb Calorimetry Worksheet.
From www.chegg.com
Solved a) Calibration i. A bomb calorimeter filled with Bomb Calorimetry Worksheet 1) a compound is burned in a bomb calorimeter that. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c6h5cooh), whose heat of. one method of generating electricity is by burning coal to heat water, which. Bomb Calorimetry Worksheet.
From www.studocu.com
Dry ABC DRY LAB A BOMB CALORIMETRY WORKSHEET A l Work Sheet Do Bomb Calorimetry Worksheet one method of generating electricity is by burning coal to heat water, which produces steam that drives an electric generator. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing. Cp (h2o) = 4.184 j / g0c h = mcp t.. Bomb Calorimetry Worksheet.
From www.scribd.com
Bomb Calorimetry Practice Problems Bomb Calorimetry Worksheet Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. 1) a compound is burned in a bomb calorimeter that. explain why this is, based on your knowledge of how bomb calorimetry works. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. Cp. Bomb Calorimetry Worksheet.
From www.studocu.com
Bomb Calorimetry Worksheets for lab BOMB CALORIMETRY LAB WORKSHEETS Bomb Calorimetry Worksheet The experimental data ignores the mass and. explain why this is, based on your knowledge of how bomb calorimetry works. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. 1) a compound is burned in a bomb calorimeter that. 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing. . Bomb Calorimetry Worksheet.
From studymediabachmeier.z21.web.core.windows.net
Heat And Calorimetry Worksheets Bomb Calorimetry Worksheet 1) a compound is burned in a bomb calorimeter that. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing. . Bomb Calorimetry Worksheet.
From byjus.com
What is bomb calorimeter? Bomb Calorimetry Worksheet 1) a compound is burned in a bomb calorimeter that. 1) if i burn 0.315 moles of hexane (c6h14) in a bomb calorimeter containing. solving bomb calorimeter problems a 1.000 g sample of octane (c 8h18) is burned in a bomb calorimeter containing 1200. one method of generating electricity is by burning coal to heat water, which produces. Bomb Calorimetry Worksheet.