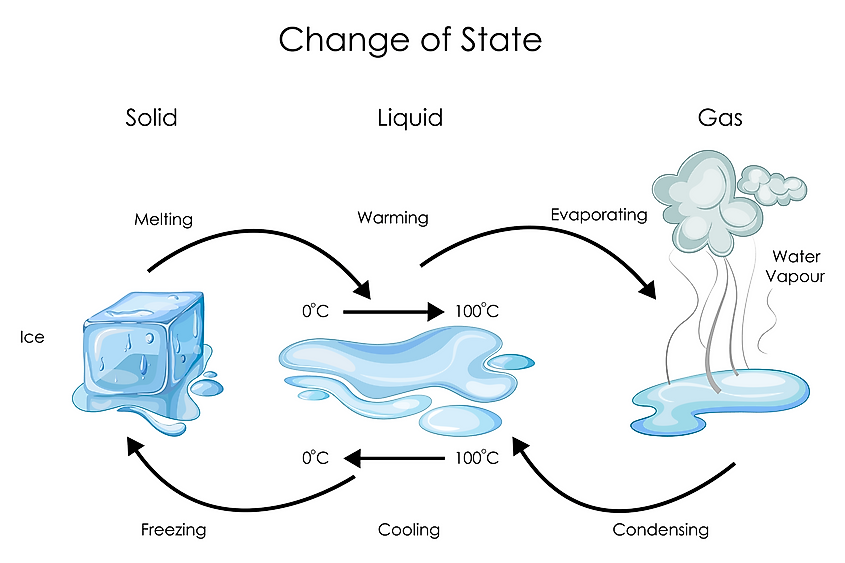
Water Melting Boiling And Freezing Point . first, at a substance’s melting point or boiling point, two phases can exist simultaneously. The melting point depends slightly on pressure, so there is not a single temperature that can be considered to be the melting point of water. Take water (h 2 o) as an. the normal boiling point of water is 100 o c because this is the temperature at which the vapor pressure of water is 760 mmhg, or 1 atm. The solid and liquid phase of water are in equilibrium at this temperature. not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. the freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature. the freezing point and melting point occur at the same temperature for a substance, but are approached from opposite directions. the melting point of water is the temperature at which it changes from solid ice into liquid water.
from schematiclistpact101.z22.web.core.windows.net
the freezing point and melting point occur at the same temperature for a substance, but are approached from opposite directions. first, at a substance’s melting point or boiling point, two phases can exist simultaneously. the freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature. Take water (h 2 o) as an. not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. The solid and liquid phase of water are in equilibrium at this temperature. The melting point depends slightly on pressure, so there is not a single temperature that can be considered to be the melting point of water. the normal boiling point of water is 100 o c because this is the temperature at which the vapor pressure of water is 760 mmhg, or 1 atm. the melting point of water is the temperature at which it changes from solid ice into liquid water.
Phase Change Diagram For Water
Water Melting Boiling And Freezing Point not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. The melting point depends slightly on pressure, so there is not a single temperature that can be considered to be the melting point of water. not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. the normal boiling point of water is 100 o c because this is the temperature at which the vapor pressure of water is 760 mmhg, or 1 atm. Take water (h 2 o) as an. The solid and liquid phase of water are in equilibrium at this temperature. first, at a substance’s melting point or boiling point, two phases can exist simultaneously. the melting point of water is the temperature at which it changes from solid ice into liquid water. the freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature. the freezing point and melting point occur at the same temperature for a substance, but are approached from opposite directions.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Water Melting Boiling And Freezing Point the normal boiling point of water is 100 o c because this is the temperature at which the vapor pressure of water is 760 mmhg, or 1 atm. the freezing point and melting point occur at the same temperature for a substance, but are approached from opposite directions. the freezing point of water describes the point at. Water Melting Boiling And Freezing Point.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation Water Melting Boiling And Freezing Point not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. first, at a substance’s melting point or boiling point, two phases can exist simultaneously. Take water (h 2 o) as an. the melting point of water is the temperature at which it changes from solid ice into liquid. Water Melting Boiling And Freezing Point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Water Melting Boiling And Freezing Point the normal boiling point of water is 100 o c because this is the temperature at which the vapor pressure of water is 760 mmhg, or 1 atm. Take water (h 2 o) as an. The solid and liquid phase of water are in equilibrium at this temperature. not all substances melt (or freeze) at 0 °c and. Water Melting Boiling And Freezing Point.
From www.gettyimages.ie
Freezing And Boiling Points In Celsius And Fahrenheit HighRes Vector Water Melting Boiling And Freezing Point the freezing point and melting point occur at the same temperature for a substance, but are approached from opposite directions. Take water (h 2 o) as an. the freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature. first, at a substance’s. Water Melting Boiling And Freezing Point.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Water Melting Boiling And Freezing Point Take water (h 2 o) as an. the freezing point and melting point occur at the same temperature for a substance, but are approached from opposite directions. the freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature. The solid and liquid phase. Water Melting Boiling And Freezing Point.
From scienceisntscary.wordpress.com
Boiling point Ease Into Science Water Melting Boiling And Freezing Point Take water (h 2 o) as an. the normal boiling point of water is 100 o c because this is the temperature at which the vapor pressure of water is 760 mmhg, or 1 atm. first, at a substance’s melting point or boiling point, two phases can exist simultaneously. the freezing point of water describes the point. Water Melting Boiling And Freezing Point.
From www.youtube.com
Freezing and Boiling Point Graph YouTube Water Melting Boiling And Freezing Point The melting point depends slightly on pressure, so there is not a single temperature that can be considered to be the melting point of water. first, at a substance’s melting point or boiling point, two phases can exist simultaneously. not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does.. Water Melting Boiling And Freezing Point.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free Water Melting Boiling And Freezing Point The melting point depends slightly on pressure, so there is not a single temperature that can be considered to be the melting point of water. Take water (h 2 o) as an. first, at a substance’s melting point or boiling point, two phases can exist simultaneously. the freezing point of water describes the point at which water transitions. Water Melting Boiling And Freezing Point.
From www.slideserve.com
PPT Physical Properties of Water Boiling Point, Melting Point and Water Melting Boiling And Freezing Point The melting point depends slightly on pressure, so there is not a single temperature that can be considered to be the melting point of water. The solid and liquid phase of water are in equilibrium at this temperature. the melting point of water is the temperature at which it changes from solid ice into liquid water. first, at. Water Melting Boiling And Freezing Point.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free Water Melting Boiling And Freezing Point The melting point depends slightly on pressure, so there is not a single temperature that can be considered to be the melting point of water. The solid and liquid phase of water are in equilibrium at this temperature. first, at a substance’s melting point or boiling point, two phases can exist simultaneously. Take water (h 2 o) as an.. Water Melting Boiling And Freezing Point.
From www.slideserve.com
PPT Physical Properties of Water Boiling Point, Melting Point and Water Melting Boiling And Freezing Point the freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature. first, at a substance’s melting point or boiling point, two phases can exist simultaneously. Take water (h 2 o) as an. the freezing point and melting point occur at the same. Water Melting Boiling And Freezing Point.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry Water Melting Boiling And Freezing Point the melting point of water is the temperature at which it changes from solid ice into liquid water. the normal boiling point of water is 100 o c because this is the temperature at which the vapor pressure of water is 760 mmhg, or 1 atm. first, at a substance’s melting point or boiling point, two phases. Water Melting Boiling And Freezing Point.
From exydxfwep.blob.core.windows.net
What Happens To The Boiling Point And Freezing Point Of Water When A Water Melting Boiling And Freezing Point the melting point of water is the temperature at which it changes from solid ice into liquid water. The solid and liquid phase of water are in equilibrium at this temperature. the normal boiling point of water is 100 o c because this is the temperature at which the vapor pressure of water is 760 mmhg, or 1. Water Melting Boiling And Freezing Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Water Melting Boiling And Freezing Point the freezing point and melting point occur at the same temperature for a substance, but are approached from opposite directions. The melting point depends slightly on pressure, so there is not a single temperature that can be considered to be the melting point of water. the freezing point of water describes the point at which water transitions from. Water Melting Boiling And Freezing Point.
From www.youtube.com
Melting Point, Boiling Point and Freezing Point Chemistry YouTube Water Melting Boiling And Freezing Point the normal boiling point of water is 100 o c because this is the temperature at which the vapor pressure of water is 760 mmhg, or 1 atm. Take water (h 2 o) as an. The solid and liquid phase of water are in equilibrium at this temperature. first, at a substance’s melting point or boiling point, two. Water Melting Boiling And Freezing Point.
From owlcation.com
What Are the Freezing, Melting, and Boiling Points of Solids, Liquids Water Melting Boiling And Freezing Point not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. the freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature. Take water (h 2 o) as an. the freezing point and melting. Water Melting Boiling And Freezing Point.
From schematiclistpact101.z22.web.core.windows.net
Phase Change Diagram For Water Water Melting Boiling And Freezing Point The solid and liquid phase of water are in equilibrium at this temperature. The melting point depends slightly on pressure, so there is not a single temperature that can be considered to be the melting point of water. the melting point of water is the temperature at which it changes from solid ice into liquid water. first, at. Water Melting Boiling And Freezing Point.
From www.youtube.com
Freezing and Boiling Point Graph YouTube Water Melting Boiling And Freezing Point first, at a substance’s melting point or boiling point, two phases can exist simultaneously. The solid and liquid phase of water are in equilibrium at this temperature. Take water (h 2 o) as an. the melting point of water is the temperature at which it changes from solid ice into liquid water. not all substances melt (or. Water Melting Boiling And Freezing Point.
From www.youtube.com
Difference between Melting point / Boiling point & Freezing point Water Melting Boiling And Freezing Point The melting point depends slightly on pressure, so there is not a single temperature that can be considered to be the melting point of water. The solid and liquid phase of water are in equilibrium at this temperature. the normal boiling point of water is 100 o c because this is the temperature at which the vapor pressure of. Water Melting Boiling And Freezing Point.
From www.pinterest.com
Boiling Point/Freezing/ Melting Point AnchorChart Teaching science Water Melting Boiling And Freezing Point first, at a substance’s melting point or boiling point, two phases can exist simultaneously. Take water (h 2 o) as an. the freezing point and melting point occur at the same temperature for a substance, but are approached from opposite directions. the melting point of water is the temperature at which it changes from solid ice into. Water Melting Boiling And Freezing Point.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii Water Melting Boiling And Freezing Point the freezing point and melting point occur at the same temperature for a substance, but are approached from opposite directions. the melting point of water is the temperature at which it changes from solid ice into liquid water. not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does.. Water Melting Boiling And Freezing Point.
From www.alamy.com
Boiling and Evaporation, Freezing and Melting Points of Water Water Melting Boiling And Freezing Point the freezing point and melting point occur at the same temperature for a substance, but are approached from opposite directions. first, at a substance’s melting point or boiling point, two phases can exist simultaneously. the melting point of water is the temperature at which it changes from solid ice into liquid water. the normal boiling point. Water Melting Boiling And Freezing Point.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock Water Melting Boiling And Freezing Point the melting point of water is the temperature at which it changes from solid ice into liquid water. The solid and liquid phase of water are in equilibrium at this temperature. The melting point depends slightly on pressure, so there is not a single temperature that can be considered to be the melting point of water. first, at. Water Melting Boiling And Freezing Point.
From www.alamy.com
Freezing melting and boiling point experiment illustration Stock Vector Water Melting Boiling And Freezing Point the normal boiling point of water is 100 o c because this is the temperature at which the vapor pressure of water is 760 mmhg, or 1 atm. The solid and liquid phase of water are in equilibrium at this temperature. The melting point depends slightly on pressure, so there is not a single temperature that can be considered. Water Melting Boiling And Freezing Point.
From 88guru.com
Melting Point of Ice and Boiling Point of Water 88Guru Water Melting Boiling And Freezing Point The solid and liquid phase of water are in equilibrium at this temperature. the freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature. the normal boiling point of water is 100 o c because this is the temperature at which the vapor. Water Melting Boiling And Freezing Point.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled Water Melting Boiling And Freezing Point The melting point depends slightly on pressure, so there is not a single temperature that can be considered to be the melting point of water. Take water (h 2 o) as an. first, at a substance’s melting point or boiling point, two phases can exist simultaneously. the normal boiling point of water is 100 o c because this. Water Melting Boiling And Freezing Point.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled Water Melting Boiling And Freezing Point the normal boiling point of water is 100 o c because this is the temperature at which the vapor pressure of water is 760 mmhg, or 1 atm. The melting point depends slightly on pressure, so there is not a single temperature that can be considered to be the melting point of water. The solid and liquid phase of. Water Melting Boiling And Freezing Point.
From www.jove.com
11369.jpg Water Melting Boiling And Freezing Point the melting point of water is the temperature at which it changes from solid ice into liquid water. the normal boiling point of water is 100 o c because this is the temperature at which the vapor pressure of water is 760 mmhg, or 1 atm. first, at a substance’s melting point or boiling point, two phases. Water Melting Boiling And Freezing Point.
From sciencenotes.org
What Is the Freezing Point of Water? Fahrenheit, Celsius, and Kelvin Water Melting Boiling And Freezing Point The melting point depends slightly on pressure, so there is not a single temperature that can be considered to be the melting point of water. Take water (h 2 o) as an. not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. the freezing point and melting point occur. Water Melting Boiling And Freezing Point.
From www.slideserve.com
PPT Physical Properties of Water Boiling Point, Melting Point and Water Melting Boiling And Freezing Point the melting point of water is the temperature at which it changes from solid ice into liquid water. not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. the freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while. Water Melting Boiling And Freezing Point.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Water Melting Boiling And Freezing Point first, at a substance’s melting point or boiling point, two phases can exist simultaneously. the melting point of water is the temperature at which it changes from solid ice into liquid water. Take water (h 2 o) as an. the freezing point of water describes the point at which water transitions from a liquid to a solid. Water Melting Boiling And Freezing Point.
From www.thoughtco.com
What Is the Freezing Point of Water? Water Melting Boiling And Freezing Point the melting point of water is the temperature at which it changes from solid ice into liquid water. the freezing point and melting point occur at the same temperature for a substance, but are approached from opposite directions. Take water (h 2 o) as an. The solid and liquid phase of water are in equilibrium at this temperature.. Water Melting Boiling And Freezing Point.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free Water Melting Boiling And Freezing Point Take water (h 2 o) as an. not all substances melt (or freeze) at 0 °c and boil (or condense) at 100 °c, like water does. the freezing point and melting point occur at the same temperature for a substance, but are approached from opposite directions. The solid and liquid phase of water are in equilibrium at this. Water Melting Boiling And Freezing Point.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Water Melting Boiling And Freezing Point the freezing point and melting point occur at the same temperature for a substance, but are approached from opposite directions. The melting point depends slightly on pressure, so there is not a single temperature that can be considered to be the melting point of water. first, at a substance’s melting point or boiling point, two phases can exist. Water Melting Boiling And Freezing Point.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube Water Melting Boiling And Freezing Point the freezing point and melting point occur at the same temperature for a substance, but are approached from opposite directions. the melting point of water is the temperature at which it changes from solid ice into liquid water. first, at a substance’s melting point or boiling point, two phases can exist simultaneously. The solid and liquid phase. Water Melting Boiling And Freezing Point.