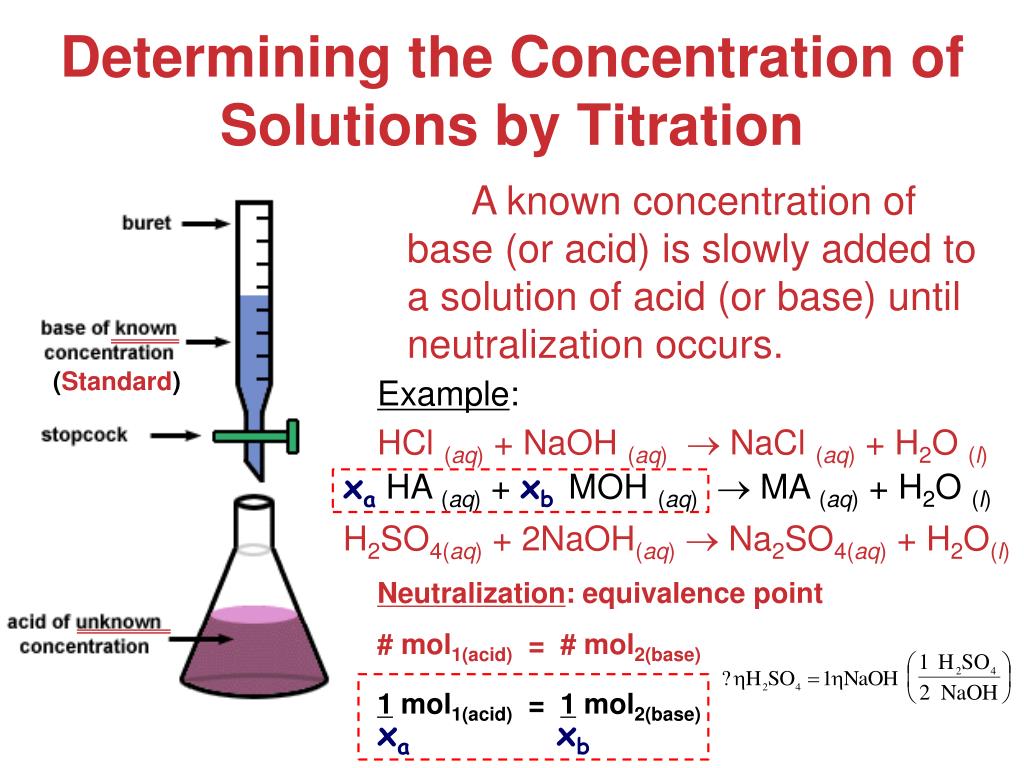
Titration Calculations Mole . First determine the moles of \(\ce{naoh}\) in the reaction. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Recall that the molarity (m) of a. The basic strategy in performing titration calculations is: First determine the moles of \(\ce{naoh}\) in the reaction. The amount of added titrant is determined from its concentration and volume: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Calculate the moles of titrant (the solution with the known concentration) needed. You may be asked to calculate the. Moles acid = moles base.
from www.slideserve.com
The basic strategy in performing titration calculations is: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. Calculate the moles of titrant (the solution with the known concentration) needed. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. First determine the moles of \(\ce{naoh}\) in the reaction. Moles acid = moles base. The amount of added titrant is determined from its concentration and volume: First determine the moles of \(\ce{naoh}\) in the reaction.
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation
Titration Calculations Mole Calculate the moles of titrant (the solution with the known concentration) needed. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Calculate the moles of titrant (the solution with the known concentration) needed. You may be asked to calculate the. Recall that the molarity (m) of a. First determine the moles of \(\ce{naoh}\) in the reaction. The amount of added titrant is determined from its concentration and volume: Moles acid = moles base. The basic strategy in performing titration calculations is: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that.
From www.youtube.com
Calculations Moles Titration Example1 YouTube Titration Calculations Mole The basic strategy in performing titration calculations is: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Recall that the molarity (m) of a. Calculate the moles of titrant (the solution with the known concentration) needed. N (mol) = c. Titration Calculations Mole.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration Titration Calculations Mole At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. First determine the moles of \(\ce{naoh}\) in the reaction. Calculate the moles of titrant (the solution with the known concentration) needed. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric.. Titration Calculations Mole.
From www.youtube.com
Back titration calculation As level mole lecture 5 YouTube Titration Calculations Mole N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Recall that the molarity (m) of a. The amount of added titrant is determined from its concentration and volume: You may be asked to calculate the. At the equivalence point in a neutralization, the moles of acid are equal. Titration Calculations Mole.
From slideplayer.com
Titration Calculation ppt download Titration Calculations Mole You may be asked to calculate the. Recall that the molarity (m) of a. First determine the moles of \(\ce{naoh}\) in the reaction. Calculate the moles of titrant (the solution with the known concentration) needed. First determine the moles of \(\ce{naoh}\) in the reaction. Moles acid = moles base. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted.. Titration Calculations Mole.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Calculations Mole First determine the moles of \(\ce{naoh}\) in the reaction. Recall that the molarity (m) of a. The amount of added titrant is determined from its concentration and volume: You may be asked to calculate the. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. Moles acid = moles. Titration Calculations Mole.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Titration Calculations Mole Calculate the moles of titrant (the solution with the known concentration) needed. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The basic strategy in performing titration calculations is: You may be asked to calculate the. First determine the moles of \(\ce{naoh}\) in the reaction. At the equivalence point in a neutralization, the moles of acid are equal. Titration Calculations Mole.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d Titration Calculations Mole From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Calculate the moles of titrant (the solution with the known concentration) needed. You may be asked to calculate the. The amount of added titrant is determined from its concentration and volume: First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\). Titration Calculations Mole.
From mungfali.com
Acid Base Titration Calculation Titration Calculations Mole First determine the moles of \(\ce{naoh}\) in the reaction. Calculate the moles of titrant (the solution with the known concentration) needed. Moles acid = moles base. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Recall that the molarity (m) of. Titration Calculations Mole.
From mungfali.com
Acid Base Titration Calculation Titration Calculations Mole The amount of added titrant is determined from its concentration and volume: The basic strategy in performing titration calculations is: Moles acid = moles base. Calculate the moles of titrant (the solution with the known concentration) needed. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. Recall that the molarity (m) of a. First determine the moles of \(\ce{naoh}\). Titration Calculations Mole.
From www.savemyexams.com
Titration Calculations Edexcel GCSE Chemistry Revision Notes 2018 Titration Calculations Mole At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. First determine the moles of \(\ce{naoh}\) in the reaction. Calculate the moles of titrant (the solution with the known concentration) needed. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. The basic strategy in performing titration calculations is: The amount of. Titration Calculations Mole.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Calculations Mole From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. Calculate the moles of titrant (the solution with the known concentration) needed. The basic strategy in performing titration calculations is: Moles acid = moles base. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The amount of added titrant is determined. Titration Calculations Mole.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Calculations Mole The basic strategy in performing titration calculations is: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Recall that the molarity (m) of a. Moles acid = moles base. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. First. Titration Calculations Mole.
From www.chegg.com
Solved Titrations Calculations 1.) Moles of NaOH 2.) Moles Titration Calculations Mole First determine the moles of \(\ce{naoh}\) in the reaction. The amount of added titrant is determined from its concentration and volume: Recall that the molarity (m) of a. Calculate the moles of titrant (the solution with the known concentration) needed. The basic strategy in performing titration calculations is: You may be asked to calculate the. At the equivalence point in. Titration Calculations Mole.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID3459790 Titration Calculations Mole First determine the moles of \(\ce{naoh}\) in the reaction. Moles acid = moles base. Calculate the moles of titrant (the solution with the known concentration) needed. The amount of added titrant is determined from its concentration and volume: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. First determine the moles of \(\ce{naoh}\) in the reaction. Recall that the. Titration Calculations Mole.
From slideplayer.com
S2/3 Chemistry Titrations. ppt download Titration Calculations Mole Moles acid = moles base. First determine the moles of \(\ce{naoh}\) in the reaction. Calculate the moles of titrant (the solution with the known concentration) needed. First determine the moles of \(\ce{naoh}\) in the reaction. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The amount of added titrant is determined. Titration Calculations Mole.
From www.coursehero.com
[Solved] Calculate the number of moles of NaOH used in your titration Titration Calculations Mole Recall that the molarity (m) of a. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Calculate the moles of titrant (the solution with the known concentration) needed. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Moles acid =. Titration Calculations Mole.
From www.youtube.com
Titration calculations, Alevel chemistry (mean titre, moles, and how Titration Calculations Mole Moles acid = moles base. The basic strategy in performing titration calculations is: Calculate the moles of titrant (the solution with the known concentration) needed. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. The amount of added titrant is determined from its concentration and volume: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. N (mol). Titration Calculations Mole.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Calculations Mole First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. First determine the moles of \(\ce{naoh}\) in the reaction. Recall that the molarity (m) of a. From the mole ratio, calculate the moles. Titration Calculations Mole.
From www.youtube.com
titration calculating moles YouTube Titration Calculations Mole Calculate the moles of titrant (the solution with the known concentration) needed. Recall that the molarity (m) of a. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. First determine the moles of \(\ce{naoh}\) in the reaction. The amount of added titrant is determined from its concentration and volume: First determine. Titration Calculations Mole.
From www.youtube.com
Concept of titration and titration’s Calculation lecture 5 (A level Titration Calculations Mole From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The amount of added titrant is determined from its concentration and volume: Moles acid = moles base. Recall that the molarity (m) of a. N (mol) = c (mol /l) * v (l) and the amount of titrant can. Titration Calculations Mole.
From www.chegg.com
Solved Titration Data Calculations Moles of Na2 S2O, in Titration Calculations Mole First determine the moles of \(\ce{naoh}\) in the reaction. Calculate the moles of titrant (the solution with the known concentration) needed. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The basic strategy in performing titration calculations is: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. The. Titration Calculations Mole.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Calculations Mole N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. First determine the moles of \(\ce{naoh}\) in the reaction. Moles. Titration Calculations Mole.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Calculations Mole The amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. First determine the moles of \(\ce{naoh}\) in the reaction. Recall that the molarity (m) of a. Moles. Titration Calculations Mole.
From ar.inspiredpencil.com
Titration Problem Examples Titration Calculations Mole First determine the moles of \(\ce{naoh}\) in the reaction. Calculate the moles of titrant (the solution with the known concentration) needed. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The amount of added titrant is determined from its concentration and volume: First determine the moles of \(\ce{naoh}\) in the reaction. Moles acid = moles base. At the. Titration Calculations Mole.
From slideplayer.com
Titrations Titration Calculations Print 1, 35, ppt download Titration Calculations Mole From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Calculate the moles of titrant (the solution with the known concentration) needed. Recall that the molarity (m) of a. First determine the moles of \(\ce{naoh}\) in the reaction. The amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l). Titration Calculations Mole.
From www.studypool.com
SOLUTION Exam with answers on volumetric analysis titration and mole Titration Calculations Mole The amount of added titrant is determined from its concentration and volume: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. Recall that the molarity (m) of a. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. Titration Calculations Mole.
From www.chegg.com
Solved Titration Data Calculations Moles of Na2 S2O3 in Titration Calculations Mole From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. Moles acid = moles base. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. The basic strategy in performing titration calculations is: The amount of added titrant is determined from its concentration and volume: First determine the moles. Titration Calculations Mole.
From www.chegg.com
Solved Titration Data Calculations Moles of Na2 S2O3 in Titration Calculations Mole Calculate the moles of titrant (the solution with the known concentration) needed. Moles acid = moles base. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. At the equivalence point in a neutralization, the moles of acid are equal. Titration Calculations Mole.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Calculations Mole Moles acid = moles base. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. First determine the moles of \(\ce{naoh}\) in the reaction. The basic strategy in performing titration calculations is: First determine the moles of \(\ce{naoh}\) in the reaction. Recall that the molarity (m) of a. From the mole ratio,. Titration Calculations Mole.
From www.studypool.com
SOLUTION Exam with answers on volumetric analysis titration and mole Titration Calculations Mole N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. First determine the moles of \(\ce{naoh}\) in the reaction. The basic strategy in performing titration calculations is: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. You may be asked. Titration Calculations Mole.
From studylib.net
Molarity, moles & mass and Volumetric titration calculations Titration Calculations Mole From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The basic strategy in performing titration calculations is: You may be asked to calculate the. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. At the equivalence point in a neutralization, the moles of acid are equal. Titration Calculations Mole.
From missmeyerscience.blogspot.com
Miss Meyer's Science Site Titration Calculations Titration Calculations Mole From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The basic strategy in performing titration calculations is: First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. You may be asked to calculate the. Moles acid = moles base. Calculate the moles of titrant (the solution with the known. Titration Calculations Mole.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Calculations Mole At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Moles acid = moles base. Recall that the molarity (m) of a. You may be asked to calculate the. The amount of added titrant is determined from its concentration and volume: Calculate the moles of titrant (the solution with the known concentration). Titration Calculations Mole.
From www.chegg.com
Solved Titration Data Calculations Moles of Na2 S2O3 in Titration Calculations Mole Calculate the moles of titrant (the solution with the known concentration) needed. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The basic strategy in performing titration calculations is: Recall that the molarity (m) of a. You. Titration Calculations Mole.
From slideplayer.com
Acid Base Titrations Lesson ppt download Titration Calculations Mole From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. The amount of added titrant is determined from its concentration and volume: Calculate the moles of titrant (the solution with the known concentration) needed. First determine the moles of \(\ce{naoh}\) in the reaction. The basic strategy in performing titration calculations is: At the equivalence point in a neutralization, the moles. Titration Calculations Mole.