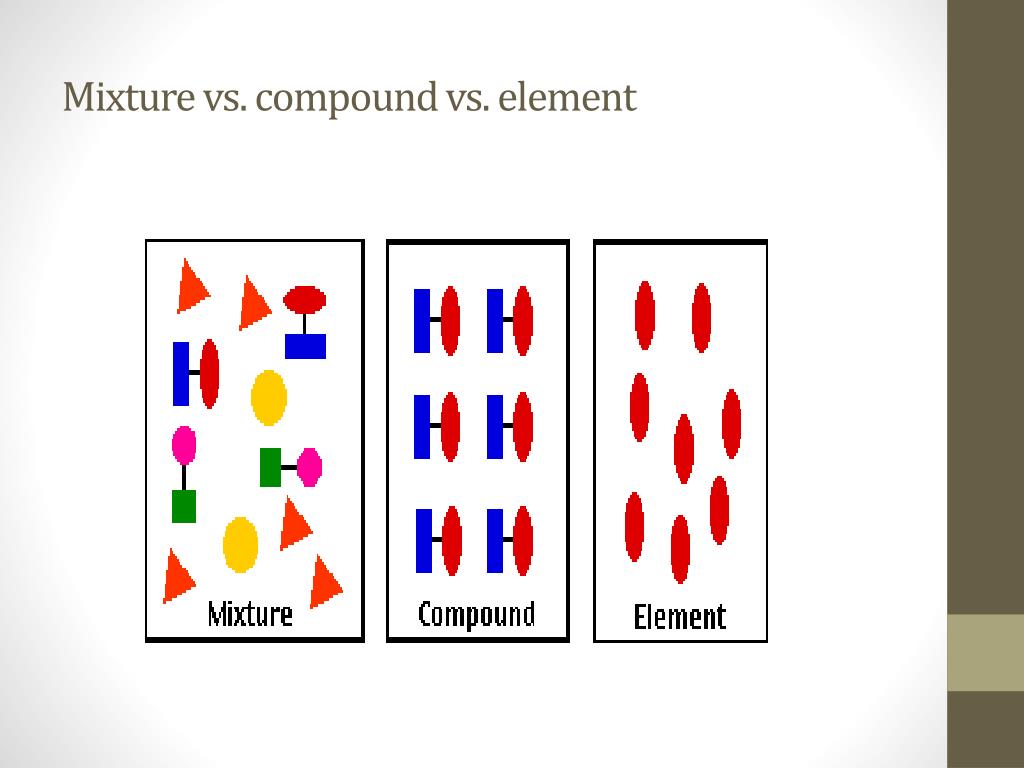
The Difference Between Elements Compounds And Mixtures . Matter can be broken down into two categories: Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. The elements in a chemical compound can only be separated by destroying the. Chemical compounds are very different from mixtures: A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that and then explain it with a diagram. A mixture is a material which has two or more types of molecules. Pure substances are further broken down into elements and compounds. A compound is made up of two more elements, the main difference being they are chemically joined, bonded, together. This page explains the difference between an element and a compound, and how both of these differ from mixtures. A compound is a pure substance which contains identical molecules with two or more types of atomic core.
from www.slideserve.com
Chemical compounds are very different from mixtures: The elements in a chemical compound can only be separated by destroying the. A compound is made up of two more elements, the main difference being they are chemically joined, bonded, together. Pure substances are further broken down into elements and compounds. A compound is a pure substance which contains identical molecules with two or more types of atomic core. A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that and then explain it with a diagram. Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. Matter can be broken down into two categories: A mixture is a material which has two or more types of molecules. This page explains the difference between an element and a compound, and how both of these differ from mixtures.
PPT Elements, Compounds, & Mixtures PowerPoint Presentation, free
The Difference Between Elements Compounds And Mixtures Chemical compounds are very different from mixtures: A mixture is a material which has two or more types of molecules. A compound is made up of two more elements, the main difference being they are chemically joined, bonded, together. The elements in a chemical compound can only be separated by destroying the. Matter can be broken down into two categories: A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that and then explain it with a diagram. Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. Chemical compounds are very different from mixtures: A compound is a pure substance which contains identical molecules with two or more types of atomic core. This page explains the difference between an element and a compound, and how both of these differ from mixtures. Pure substances are further broken down into elements and compounds.
From www.youtube.com
Difference between an and a Mixture YouTube The Difference Between Elements Compounds And Mixtures A compound is a pure substance which contains identical molecules with two or more types of atomic core. The elements in a chemical compound can only be separated by destroying the. A compound is made up of two more elements, the main difference being they are chemically joined, bonded, together. Chemical compounds are very different from mixtures: Clearly, these definitions. The Difference Between Elements Compounds And Mixtures.
From www.dreamstime.com
Elements, Mixtures, and Compounds Compared Stock Vector Illustration The Difference Between Elements Compounds And Mixtures A compound is made up of two more elements, the main difference being they are chemically joined, bonded, together. The elements in a chemical compound can only be separated by destroying the. A mixture is a material which has two or more types of molecules. Chemical compounds are very different from mixtures: Pure substances are further broken down into elements. The Difference Between Elements Compounds And Mixtures.
From www.slideserve.com
PPT Physical Science PowerPoint Presentation ID2132853 The Difference Between Elements Compounds And Mixtures This page explains the difference between an element and a compound, and how both of these differ from mixtures. A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that and then explain it with a diagram. Clearly, these definitions only apply. The Difference Between Elements Compounds And Mixtures.
From www.vrogue.co
The Difference Between Elements Compounds And Mixture vrogue.co The Difference Between Elements Compounds And Mixtures A compound is made up of two more elements, the main difference being they are chemically joined, bonded, together. Chemical compounds are very different from mixtures: Pure substances are further broken down into elements and compounds. The elements in a chemical compound can only be separated by destroying the. Matter can be broken down into two categories: Clearly, these definitions. The Difference Between Elements Compounds And Mixtures.
From www.shutterstock.com
Different Between Elements Compounds Mixtures Example Stock The Difference Between Elements Compounds And Mixtures Matter can be broken down into two categories: This page explains the difference between an element and a compound, and how both of these differ from mixtures. The elements in a chemical compound can only be separated by destroying the. Chemical compounds are very different from mixtures: Clearly, these definitions only apply to molecular materials, and will need to be. The Difference Between Elements Compounds And Mixtures.
From www.dreamstime.com
Mixtures of Both Elements and Compounds Stock Vector Illustration of The Difference Between Elements Compounds And Mixtures Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. A compound is made up of two more elements, the main difference being they are chemically joined, bonded, together. Chemical compounds are very different from mixtures: This page explains the difference between an element and a compound, and how both. The Difference Between Elements Compounds And Mixtures.
From www.youtube.com
Difference and Similarities between Element and Compound Chemistry The Difference Between Elements Compounds And Mixtures Matter can be broken down into two categories: Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. Pure substances are further broken down into elements and compounds. Chemical compounds are very different from mixtures: A compound is made up of two more elements, the main difference being they are. The Difference Between Elements Compounds And Mixtures.
From quizlet.com
Elements, Compounds, and Mixtures Diagram Quizlet The Difference Between Elements Compounds And Mixtures The elements in a chemical compound can only be separated by destroying the. Pure substances are further broken down into elements and compounds. A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that and then explain it with a diagram. A. The Difference Between Elements Compounds And Mixtures.
From www.slideserve.com
PPT Elements, Compounds and Mixtures PowerPoint Presentation, free The Difference Between Elements Compounds And Mixtures This page explains the difference between an element and a compound, and how both of these differ from mixtures. Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. Chemical compounds are very different from mixtures: A mixture is a material which has two or more types of molecules. Pure. The Difference Between Elements Compounds And Mixtures.
From techdiagrammer.com
Understanding the Key Differences between Elements, Compounds, and The Difference Between Elements Compounds And Mixtures Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. A compound is made up of two more elements, the main difference being they are chemically joined, bonded, together. The elements in a chemical compound can only be separated by destroying the. This page explains the difference between an element. The Difference Between Elements Compounds And Mixtures.
From techdiagrammer.com
Understanding the Key Differences between Elements, Compounds, and The Difference Between Elements Compounds And Mixtures A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that and then explain it with a diagram. Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. This page explains the. The Difference Between Elements Compounds And Mixtures.
From brainly.in
images of elements compounds and mixtures differences Brainly.in The Difference Between Elements Compounds And Mixtures Matter can be broken down into two categories: Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. A compound is made up of two more elements, the main difference being they are chemically joined, bonded, together. A mixture is a material which has two or more types of molecules.. The Difference Between Elements Compounds And Mixtures.
From www.pinterest.com
Element, Mixture, Compound Activity Take two, Chimie The Difference Between Elements Compounds And Mixtures This page explains the difference between an element and a compound, and how both of these differ from mixtures. A mixture is a material which has two or more types of molecules. A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with. The Difference Between Elements Compounds And Mixtures.
From www.slideserve.com
PPT Elements, Compounds & Mixtures PowerPoint Presentation ID4023965 The Difference Between Elements Compounds And Mixtures Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. A compound is a pure substance which contains identical molecules with two or more types of atomic core. The elements in a chemical compound can only be separated by destroying the. A compound is made up of two more elements,. The Difference Between Elements Compounds And Mixtures.
From www.youtube.com
Difference Between Mixture And Compound?Class Series YouTube The Difference Between Elements Compounds And Mixtures Chemical compounds are very different from mixtures: A mixture is a material which has two or more types of molecules. Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. The elements in a chemical compound can only be separated by destroying the. Matter can be broken down into two. The Difference Between Elements Compounds And Mixtures.
From www.slideserve.com
PPT Elements, Compounds and Mixtures PowerPoint Presentation, free The Difference Between Elements Compounds And Mixtures A compound is a pure substance which contains identical molecules with two or more types of atomic core. The elements in a chemical compound can only be separated by destroying the. A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that. The Difference Between Elements Compounds And Mixtures.
From www.expii.com
Homogeneous vs. Heterogeneous Mixtures — A Comparison Expii The Difference Between Elements Compounds And Mixtures Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. Chemical compounds are very different from mixtures: The elements in a chemical compound can only be separated by destroying the. A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned. The Difference Between Elements Compounds And Mixtures.
From catkelbelbayabz.weebly.com
Chapter 3 20132014 Chemistry The Difference Between Elements Compounds And Mixtures A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that and then explain it with a diagram. Chemical compounds are very different from mixtures: The elements in a chemical compound can only be separated by destroying the. This page explains the. The Difference Between Elements Compounds And Mixtures.
From www.youtube.com
Difference Between Mixture and Compound Daily Life Examples The Difference Between Elements Compounds And Mixtures A mixture is a material which has two or more types of molecules. A compound is a pure substance which contains identical molecules with two or more types of atomic core. A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that. The Difference Between Elements Compounds And Mixtures.
From www.slideshare.net
Elements, Compounds, Mixtures The Difference Between Elements Compounds And Mixtures A compound is a pure substance which contains identical molecules with two or more types of atomic core. Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. Matter can be broken down into two categories: Chemical compounds are very different from mixtures: Pure substances are further broken down into. The Difference Between Elements Compounds And Mixtures.
From eduforkid.com
What Is The Difference Between Elements And Compounds Explain With The Difference Between Elements Compounds And Mixtures Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. A mixture is a material which has two or more types of molecules. A compound is made up of two more elements, the main difference being they are chemically joined, bonded, together. This page explains the difference between an element. The Difference Between Elements Compounds And Mixtures.
From www.slideshare.net
Elements, Compounds, Mixtures The Difference Between Elements Compounds And Mixtures The elements in a chemical compound can only be separated by destroying the. A mixture is a material which has two or more types of molecules. A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that and then explain it with. The Difference Between Elements Compounds And Mixtures.
From www.majordifferences.com
6 Differences between Compounds and Mixtures with examples MD The Difference Between Elements Compounds And Mixtures A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that and then explain it with a diagram. Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. A compound is made. The Difference Between Elements Compounds And Mixtures.
From armanitaromartin.blogspot.com
Difference Between Mixture and Compound ArmanitaroMartin The Difference Between Elements Compounds And Mixtures Chemical compounds are very different from mixtures: The elements in a chemical compound can only be separated by destroying the. A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that and then explain it with a diagram. Clearly, these definitions only. The Difference Between Elements Compounds And Mixtures.
From www.pinterest.com
GCSE CHEMISTRY Atoms, Elements and Compounds Mixtures COMPLETE The Difference Between Elements Compounds And Mixtures Chemical compounds are very different from mixtures: A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that and then explain it with a diagram. A mixture is a material which has two or more types of molecules. Pure substances are further. The Difference Between Elements Compounds And Mixtures.
From stock.adobe.com
illustration of chemistry, Pure Substances and Mixtures, element The Difference Between Elements Compounds And Mixtures A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that and then explain it with a diagram. Pure substances are further broken down into elements and compounds. A compound is a pure substance which contains identical molecules with two or more. The Difference Between Elements Compounds And Mixtures.
From www.slideserve.com
PPT Elements, Compounds, & Mixtures PowerPoint Presentation, free The Difference Between Elements Compounds And Mixtures This page explains the difference between an element and a compound, and how both of these differ from mixtures. A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that and then explain it with a diagram. A mixture is a material. The Difference Between Elements Compounds And Mixtures.
From chemistryclinic.co.uk
1.8 Elements, compounds, mixtures Chemistry The Difference Between Elements Compounds And Mixtures Chemical compounds are very different from mixtures: Pure substances are further broken down into elements and compounds. A compound is made up of two more elements, the main difference being they are chemically joined, bonded, together. Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. A compound is a. The Difference Between Elements Compounds And Mixtures.
From acamrmicheal.weebly.com
Properties of Mixtures ACA Grade 8 Science The Difference Between Elements Compounds And Mixtures A compound is a pure substance which contains identical molecules with two or more types of atomic core. Matter can be broken down into two categories: Chemical compounds are very different from mixtures: Pure substances are further broken down into elements and compounds. This page explains the difference between an element and a compound, and how both of these differ. The Difference Between Elements Compounds And Mixtures.
From mavink.com
Element Compound And Mixture Examples The Difference Between Elements Compounds And Mixtures Pure substances are further broken down into elements and compounds. A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that and then explain it with a diagram. This page explains the difference between an element and a compound, and how both. The Difference Between Elements Compounds And Mixtures.
From techdiagrammer.com
Understanding the Key Differences between Elements, Compounds, and The Difference Between Elements Compounds And Mixtures This page explains the difference between an element and a compound, and how both of these differ from mixtures. The elements in a chemical compound can only be separated by destroying the. A compound is a pure substance which contains identical molecules with two or more types of atomic core. Chemical compounds are very different from mixtures: A mixture is. The Difference Between Elements Compounds And Mixtures.
From www.youtube.com
Compound and Mixture Class 9 What is the Difference between Compound The Difference Between Elements Compounds And Mixtures This page explains the difference between an element and a compound, and how both of these differ from mixtures. A compound is a pure substance which contains identical molecules with two or more types of atomic core. Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. The elements in. The Difference Between Elements Compounds And Mixtures.
From www.tes.com
Elements, compounds and mixtures Teaching Resources The Difference Between Elements Compounds And Mixtures Matter can be broken down into two categories: A mixture is a material which has two or more types of molecules. Pure substances are further broken down into elements and compounds. Chemical compounds are very different from mixtures: The elements in a chemical compound can only be separated by destroying the. This page explains the difference between an element and. The Difference Between Elements Compounds And Mixtures.
From www.youtube.com
Chemistry 9th Chapter 1 Difference between Compound and Mixture The Difference Between Elements Compounds And Mixtures Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later. This page explains the difference between an element and a compound, and how both of these differ from mixtures. A compound is made up of two more elements, the main difference being they are chemically joined, bonded, together. The elements. The Difference Between Elements Compounds And Mixtures.
From ivyroses.com
Elements, Mixtures and Compounds vs Atoms and Molecules School Chemistry The Difference Between Elements Compounds And Mixtures Pure substances are further broken down into elements and compounds. A compound is different to both elements and mixtures and we recommend you have a simple and clear definition learned in your head so you can start with that and then explain it with a diagram. This page explains the difference between an element and a compound, and how both. The Difference Between Elements Compounds And Mixtures.