Bacterial Detection In Platelets . The assays are optimal for rapidly growing bacteria, which are. Rapid detection methods improve pc safety regarding bacterial contamination. Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an appropriately. In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. The detection of bacteria in platelet products can be improved with a combination of rapid testing and bacterial cultures that involve. The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and.
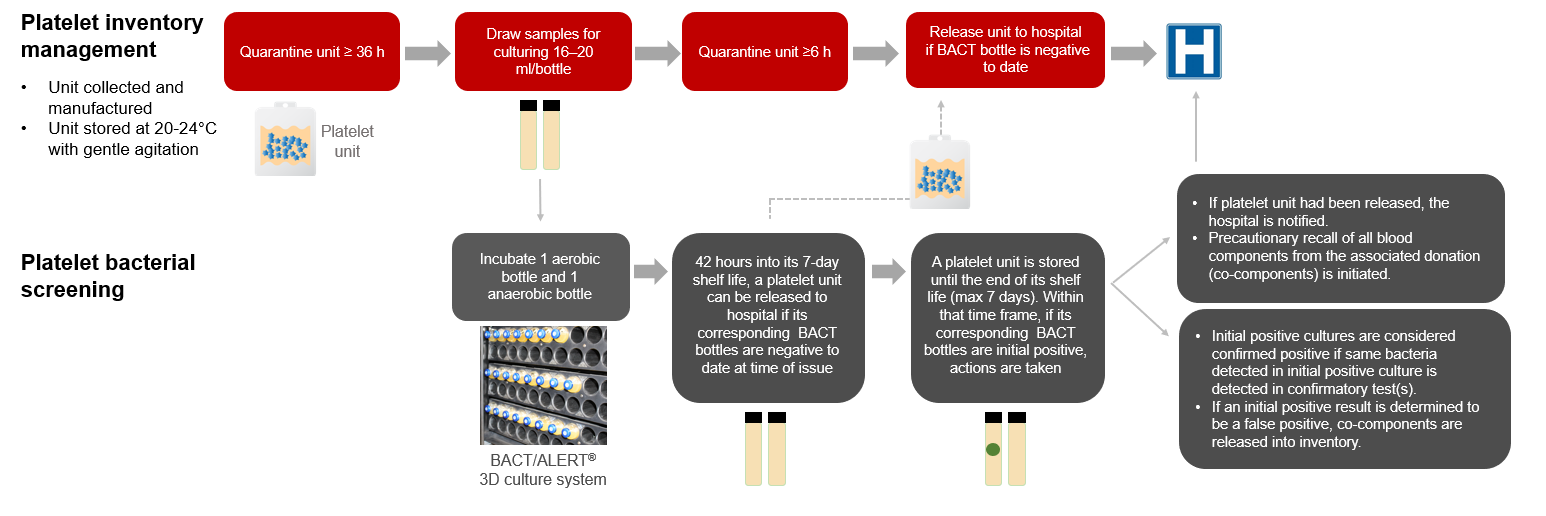
from professionaleducation.blood.ca
The assays are optimal for rapidly growing bacteria, which are. Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. The detection of bacteria in platelet products can be improved with a combination of rapid testing and bacterial cultures that involve. Rapid detection methods improve pc safety regarding bacterial contamination. The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an appropriately. In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and.
FAQ Canadian Blood Services platelet bacterial screening
Bacterial Detection In Platelets The assays are optimal for rapidly growing bacteria, which are. The assays are optimal for rapidly growing bacteria, which are. This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. Rapid detection methods improve pc safety regarding bacterial contamination. Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an appropriately. The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. The detection of bacteria in platelet products can be improved with a combination of rapid testing and bacterial cultures that involve. Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating.
From www.slideserve.com
PPT Bacterial Contamination in Blood Products Risks, Prevention and Bacterial Detection In Platelets The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an appropriately. Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. In the guidance document, the fda identified several testing strategies that would. Bacterial Detection In Platelets.
From www.slideserve.com
PPT Rapid Tests for Detection of Bacterial Contamination of Platelets Bacterial Detection In Platelets Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an appropriately. This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. The detection of bacteria in platelet products can. Bacterial Detection In Platelets.
From clpmag.com
Platelet Test Detects Bacterial Contamination Clinical Lab Products Bacterial Detection In Platelets Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. Rapid detection methods improve pc safety regarding bacterial contamination. This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. The detection of bacteria in platelet products can be improved with a combination of rapid testing. Bacterial Detection In Platelets.
From www.jthjournal.org
Platelets kill bacteria by bridging innate and adaptive immunity via Bacterial Detection In Platelets Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. Rapid detection methods improve pc safety regarding bacterial contamination. The detection of bacteria in platelet products can be improved with a combination of rapid testing. Bacterial Detection In Platelets.
From www.slideserve.com
PPT Bacterial Testing of Platelet Components 2008 Update PowerPoint Bacterial Detection In Platelets Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an appropriately. In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. This narrative review provides an overview of the problem and an update on. Bacterial Detection In Platelets.
From www.ahajournals.org
Role of Platelets in Detection and Regulation of Infection Bacterial Detection In Platelets Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an appropriately. This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. The detection of bacteria in platelet products can be improved with a combination of rapid testing and bacterial cultures that involve. In the guidance. Bacterial Detection In Platelets.
From www.annclinlabsci.org
Rapid Detection of Bacterial Contamination of PlateletRich Plasma Bacterial Detection In Platelets This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. In the guidance document, the fda identified several testing strategies. Bacterial Detection In Platelets.
From www.veraxbiomedical.com
Platelet Bacterial Contamination Detection Verax Biomedical Bacterial Detection In Platelets The assays are optimal for rapidly growing bacteria, which are. The detection of bacteria in platelet products can be improved with a combination of rapid testing and bacterial cultures that involve. The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. Rapid detection methods improve pc safety regarding bacterial contamination. Current bacterial screening technology is. Bacterial Detection In Platelets.
From www.slideserve.com
PPT Rapid Tests for Detection of Bacterial Contamination of Platelets Bacterial Detection In Platelets The assays are optimal for rapidly growing bacteria, which are. In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. This narrative review provides. Bacterial Detection In Platelets.
From www.slideserve.com
PPT Bacterial Testing of Platelet Components 2008 Update PowerPoint Bacterial Detection In Platelets Rapid detection methods improve pc safety regarding bacterial contamination. This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. The detection of bacteria in platelet products can be improved with a combination of rapid testing. Bacterial Detection In Platelets.
From www.veraxbiomedical.com
Platelet Bacterial Contamination Detection Verax Biomedical Bacterial Detection In Platelets In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. Rapid detection methods improve pc safety regarding bacterial contamination. The detection of bacteria in platelet. Bacterial Detection In Platelets.
From www.mdpi.com
Biosensors Free FullText Rapid Bacteria Detection from Patients Bacterial Detection In Platelets Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. The assays are optimal for rapidly growing bacteria, which are. Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an appropriately. Rapid detection methods improve pc safety regarding bacterial contamination. In the guidance document, the fda identified several. Bacterial Detection In Platelets.
From www.annclinlabsci.org
Rapid Detection of Bacterial Contamination of PlateletRich Plasma Bacterial Detection In Platelets Rapid detection methods improve pc safety regarding bacterial contamination. In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. The assays are optimal for rapidly growing bacteria, which are. Current bacterial screening technology is useful for blocking platelet units that. Bacterial Detection In Platelets.
From www.academia.edu
(PDF) VALIDATION OF NEW ASSAY FOR BACTERIAL DETECTION OF RANDOM DONOR Bacterial Detection In Platelets Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would. Bacterial Detection In Platelets.
From www.slideserve.com
PPT Bacterial Contamination in Blood Products Risks, Prevention and Bacterial Detection In Platelets The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. The detection of bacteria in platelet products can be improved with a combination of rapid testing and bacterial cultures that involve. Returning to platelet storage beyond 5 days and. Bacterial Detection In Platelets.
From www.mdpi.com
Cells Free FullText Platelets, Bacterial Adhesins and the Pneumococcus Bacterial Detection In Platelets Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an appropriately. Rapid detection methods improve pc safety regarding bacterial contamination. The assays are. Bacterial Detection In Platelets.
From www.slideserve.com
PPT Bacterial Contamination in Blood Products Risks, Prevention and Bacterial Detection In Platelets Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. Rapid detection methods improve pc safety regarding bacterial contamination. Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an. Bacterial Detection In Platelets.
From www.academia.edu
(PDF) Bacterial detection in apheresis platelets blood systems Bacterial Detection In Platelets The assays are optimal for rapidly growing bacteria, which are. Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an appropriately. The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. This narrative review provides an overview of the problem and an update on strategies for the prevention, detection,. Bacterial Detection In Platelets.
From www.slideserve.com
PPT Bacterial Testing of Platelet Components 2008 Update PowerPoint Bacterial Detection In Platelets The detection of bacteria in platelet products can be improved with a combination of rapid testing and bacterial cultures that involve. The assays are optimal for rapidly growing bacteria, which are. In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use. Bacterial Detection In Platelets.
From www.slideserve.com
PPT Bacterial Contamination in Blood Products Risks, Prevention and Bacterial Detection In Platelets In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. This narrative review provides an overview of the problem and an update on strategies for. Bacterial Detection In Platelets.
From www.slideserve.com
PPT Bacterial Testing of Platelet Components 2008 Update PowerPoint Bacterial Detection In Platelets This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. The assays are optimal for rapidly growing bacteria, which are. In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. The. Bacterial Detection In Platelets.
From www.slideserve.com
PPT Bacterial Testing of Platelet Components 2008 Update PowerPoint Bacterial Detection In Platelets This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an appropriately. The assays are optimal for rapidly growing bacteria, which are. The detection of bacteria in platelet products can be improved with a combination of. Bacterial Detection In Platelets.
From www.slideserve.com
PPT Bacterial Contamination in Blood Products Risks, Prevention and Bacterial Detection In Platelets The detection of bacteria in platelet products can be improved with a combination of rapid testing and bacterial cultures that involve. This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. Current bacterial screening technology is. Bacterial Detection In Platelets.
From www.slideserve.com
PPT Bacterial Testing of Platelet Components 2008 Update PowerPoint Bacterial Detection In Platelets Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an appropriately. The assays are optimal for rapidly growing bacteria, which are. The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. Rapid detection. Bacterial Detection In Platelets.
From www.mdpi.com
Cells Free FullText Platelets, Bacterial Adhesins and the Pneumococcus Bacterial Detection In Platelets This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. The detection of bacteria in platelet products can be improved with a combination of rapid testing and bacterial cultures that involve. In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units. Bacterial Detection In Platelets.
From www.slideserve.com
PPT Bacterial Contamination in Blood Products Risks, Prevention and Bacterial Detection In Platelets Rapid detection methods improve pc safety regarding bacterial contamination. Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an appropriately. In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. The detection of bacteria. Bacterial Detection In Platelets.
From professionaleducation.blood.ca
FAQ Canadian Blood Services platelet bacterial screening Bacterial Detection In Platelets In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. Current bacterial screening technology is useful for blocking platelet units that. Bacterial Detection In Platelets.
From www.veraxbiomedical.com
Platelet Bacterial Contamination Detection Verax Biomedical Bacterial Detection In Platelets The assays are optimal for rapidly growing bacteria, which are. Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. The detection of bacteria in platelet products can be improved with a combination of rapid testing and bacterial cultures that involve. Rapid detection methods improve pc safety regarding bacterial contamination. Returning to platelet storage. Bacterial Detection In Platelets.
From www.veraxbiomedical.com
Platelet Bacterial Contamination Detection Verax Biomedical Bacterial Detection In Platelets In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. Returning to platelet storage beyond 5 days and allowing prestorage pooling of platelets would require an appropriately. Current bacterial screening technology is useful for blocking platelet units that contain high. Bacterial Detection In Platelets.
From www.slideserve.com
PPT IL RISCHIO TRASFUSIONALE PowerPoint Presentation, free download Bacterial Detection In Platelets The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. The assays are optimal for rapidly growing bacteria, which are. This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. In the guidance document, the fda identified several testing strategies that would be acceptable to ensure. Bacterial Detection In Platelets.
From www.slideserve.com
PPT Bacterial Contamination in Blood Products Risks, Prevention and Bacterial Detection In Platelets Rapid detection methods improve pc safety regarding bacterial contamination. In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. The detection of bacteria in platelet. Bacterial Detection In Platelets.
From www.veraxbiomedical.com
Platelet PGD (Pan Genera Detection) System Verax Biomedical Bacterial Detection In Platelets The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. Rapid detection methods improve pc safety regarding bacterial contamination. This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. The detection of bacteria in platelet products can be improved with a combination of rapid testing and. Bacterial Detection In Platelets.
From www.cbcspecialtyservices.org
Platelet Bacterial Detection FDA Guidance What You Need to Know Bacterial Detection In Platelets The association for the advancement of blood & biotherapies (aabb) requires blood collection and transfusion. Rapid detection methods improve pc safety regarding bacterial contamination. In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. The assays are optimal for rapidly. Bacterial Detection In Platelets.
From www.researchgate.net
Schematic of bacterial detection via a SERS sandwich strategy, in which Bacterial Detection In Platelets Current bacterial screening technology is useful for blocking platelet units that contain high levels of contaminating. The assays are optimal for rapidly growing bacteria, which are. In the guidance document, the fda identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of. The detection of bacteria. Bacterial Detection In Platelets.
From www.blood.ca
How do bacteria behave during platelet production? Canadian Blood Bacterial Detection In Platelets This narrative review provides an overview of the problem and an update on strategies for the prevention, detection, and. Rapid detection methods improve pc safety regarding bacterial contamination. The assays are optimal for rapidly growing bacteria, which are. The detection of bacteria in platelet products can be improved with a combination of rapid testing and bacterial cultures that involve. Returning. Bacterial Detection In Platelets.