Medical Device Definition By Fda . Overview of regulations for medical devices: The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Premarket notifications (510(k)), establishment registration, device listing,. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: An instrument, apparatus, implement, machine,. Exemptions from federal preemption of state and local medical device requirements: The information on this page is current as of mar 22, 2024. Fda regulates the sale of medical device products in the u.s. And monitors the safety of all regulated medical products.
from www.kolabtree.com
Fda regulates the sale of medical device products in the u.s. The information on this page is current as of mar 22, 2024. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. An instrument, apparatus, implement, machine,. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Exemptions from federal preemption of state and local medical device requirements: Premarket notifications (510(k)), establishment registration, device listing,. And monitors the safety of all regulated medical products. Overview of regulations for medical devices:
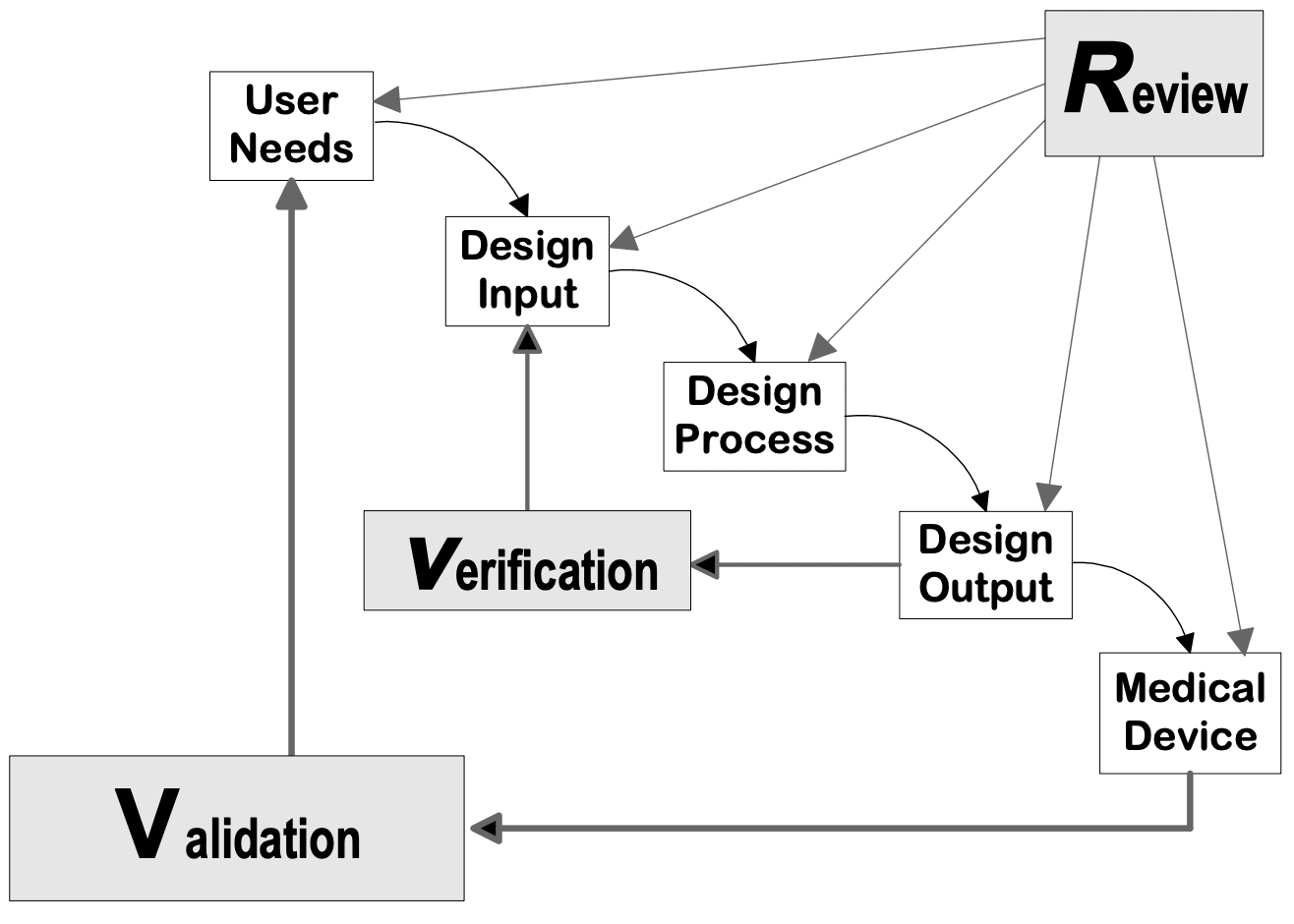
A guide to FDA Design Controls for your medical device
Medical Device Definition By Fda The information on this page is current as of mar 22, 2024. Premarket notifications (510(k)), establishment registration, device listing,. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. And monitors the safety of all regulated medical products. Fda regulates the sale of medical device products in the u.s. Overview of regulations for medical devices: Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: The information on this page is current as of mar 22, 2024. Exemptions from federal preemption of state and local medical device requirements: An instrument, apparatus, implement, machine,.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Device Definition By Fda Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Exemptions from federal preemption of state and local medical device requirements: Overview of regulations for medical devices: An instrument, apparatus, implement, machine,. Fda regulates the sale of medical device products in the u.s. The food and drug administration (fda) has established classifications for approximately 1,700. Medical Device Definition By Fda.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Medical Device Definition By Fda The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. And monitors the safety of all regulated medical products. The information on this page is current as of mar 22, 2024. Premarket notifications (510(k)), establishment registration, device listing,. Exemptions from federal preemption of state and local medical device requirements: Overview of regulations. Medical Device Definition By Fda.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Medical Device Definition By Fda An instrument, apparatus, implement, machine,. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Overview of regulations for medical devices: Fda regulates the sale of medical device products in the u.s. The information on this page is current as of mar 22, 2024. And monitors the safety of all regulated medical products. The food. Medical Device Definition By Fda.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Medical Device Definition By Fda And monitors the safety of all regulated medical products. Fda regulates the sale of medical device products in the u.s. Overview of regulations for medical devices: The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Exemptions from federal preemption of state and local medical device requirements: Section 201(h) of the food,. Medical Device Definition By Fda.
From premier-research.com
Medical Device Trials What You Need to Know About U.S. Regulations Medical Device Definition By Fda Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Exemptions from federal preemption of state and local medical device requirements: The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Overview of regulations for medical devices: And monitors the safety of all regulated medical products. Premarket. Medical Device Definition By Fda.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Device Definition By Fda The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Fda regulates the sale of medical device products in the u.s. The information on this page is current as of mar 22, 2024. Premarket notifications (510(k)), establishment registration, device listing,. Exemptions from federal preemption of state and local medical device requirements: Section. Medical Device Definition By Fda.
From www.presentationeze.com
FDA medical device classification PresentationEZE Medical Device Definition By Fda The information on this page is current as of mar 22, 2024. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Overview of regulations for medical devices: An instrument, apparatus, implement, machine,. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Fda regulates the sale. Medical Device Definition By Fda.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Definition By Fda Overview of regulations for medical devices: And monitors the safety of all regulated medical products. An instrument, apparatus, implement, machine,. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Fda regulates the sale of medical device products in the u.s. The information on this page is current as of mar 22, 2024. Premarket notifications. Medical Device Definition By Fda.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Medical Device Definition By Fda And monitors the safety of all regulated medical products. The information on this page is current as of mar 22, 2024. Overview of regulations for medical devices: An instrument, apparatus, implement, machine,. Fda regulates the sale of medical device products in the u.s. Exemptions from federal preemption of state and local medical device requirements: The food and drug administration (fda). Medical Device Definition By Fda.
From healthtrustpg.com
FDA Approval Update HealthTrust Performance Improvement For Healthcare Medical Device Definition By Fda Exemptions from federal preemption of state and local medical device requirements: The information on this page is current as of mar 22, 2024. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. And monitors the safety of all regulated medical products. Overview of regulations for medical devices: Fda regulates the sale. Medical Device Definition By Fda.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Definition By Fda Premarket notifications (510(k)), establishment registration, device listing,. And monitors the safety of all regulated medical products. An instrument, apparatus, implement, machine,. Fda regulates the sale of medical device products in the u.s. Exemptions from federal preemption of state and local medical device requirements: The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. Medical Device Definition By Fda.
From barcode-labels.com
Medical Device Labels Electronic Imaging Materials Medical Device Definition By Fda And monitors the safety of all regulated medical products. Overview of regulations for medical devices: Fda regulates the sale of medical device products in the u.s. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: The information on this page is current as of mar 22, 2024. Exemptions from federal preemption of state and. Medical Device Definition By Fda.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Medical Device Definition By Fda An instrument, apparatus, implement, machine,. The information on this page is current as of mar 22, 2024. Overview of regulations for medical devices: Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Fda regulates the sale of medical device products in the u.s. Premarket notifications (510(k)), establishment registration, device listing,. Exemptions from federal preemption. Medical Device Definition By Fda.
From www.youtube.com
FDA Regulation of Medical Devices and Software/Apps YouTube Medical Device Definition By Fda The information on this page is current as of mar 22, 2024. Exemptions from federal preemption of state and local medical device requirements: Premarket notifications (510(k)), establishment registration, device listing,. Overview of regulations for medical devices: And monitors the safety of all regulated medical products. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types. Medical Device Definition By Fda.
From medium.com
The 3 FDA medical device classes [differences and examples explained Medical Device Definition By Fda And monitors the safety of all regulated medical products. Exemptions from federal preemption of state and local medical device requirements: Premarket notifications (510(k)), establishment registration, device listing,. Fda regulates the sale of medical device products in the u.s. An instrument, apparatus, implement, machine,. The information on this page is current as of mar 22, 2024. The food and drug administration. Medical Device Definition By Fda.
From vem-medical.com
Medical Device Manufacturing Medical Device Definition By Fda And monitors the safety of all regulated medical products. Fda regulates the sale of medical device products in the u.s. An instrument, apparatus, implement, machine,. Exemptions from federal preemption of state and local medical device requirements: The information on this page is current as of mar 22, 2024. Overview of regulations for medical devices: Premarket notifications (510(k)), establishment registration, device. Medical Device Definition By Fda.
From www.medtechimpact.com
FDA Focuses on Medical Device Safety • Medtech Impact On Wellness Medical Device Definition By Fda The information on this page is current as of mar 22, 2024. Premarket notifications (510(k)), establishment registration, device listing,. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Overview of regulations for medical devices: Fda regulates the sale of medical device products in the u.s. Section 201(h) of the food, drug. Medical Device Definition By Fda.
From www.slideserve.com
PPT FDA Regulation of In Vitro Diagnostic Tests PowerPoint Medical Device Definition By Fda Overview of regulations for medical devices: The information on this page is current as of mar 22, 2024. And monitors the safety of all regulated medical products. Premarket notifications (510(k)), establishment registration, device listing,. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: An instrument, apparatus, implement, machine,. Fda regulates the sale of medical. Medical Device Definition By Fda.
From www.slideserve.com
PPT Risk Assessments Patient Safety and Innovation PowerPoint Medical Device Definition By Fda Overview of regulations for medical devices: Exemptions from federal preemption of state and local medical device requirements: Fda regulates the sale of medical device products in the u.s. Premarket notifications (510(k)), establishment registration, device listing,. The information on this page is current as of mar 22, 2024. An instrument, apparatus, implement, machine,. The food and drug administration (fda) has established. Medical Device Definition By Fda.
From www.greenlight.guru
Am I Complying with FDA Medical Device Labeling Requirements? Medical Device Definition By Fda Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: An instrument, apparatus, implement, machine,. The information on this page is current as of mar 22, 2024. Premarket notifications (510(k)), establishment registration, device listing,. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. And monitors the. Medical Device Definition By Fda.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Definition By Fda Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: And monitors the safety of all regulated medical products. The information on this page is current as of mar 22, 2024. An instrument, apparatus, implement, machine,. Premarket notifications (510(k)), establishment registration, device listing,. The food and drug administration (fda) has established classifications for approximately 1,700. Medical Device Definition By Fda.
From www.linkedin.com
FDA Regulatory Pathways for Medical Devices Medical Device Definition By Fda Exemptions from federal preemption of state and local medical device requirements: Overview of regulations for medical devices: Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Premarket notifications (510(k)), establishment registration, device listing,. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Fda regulates the. Medical Device Definition By Fda.
From spyro-soft.com
EU MDR vs FDA what are the main differences and similarities? Medical Device Definition By Fda And monitors the safety of all regulated medical products. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Premarket notifications (510(k)), establishment registration, device listing,. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Exemptions from federal preemption of state and local medical device requirements:. Medical Device Definition By Fda.
From www.pinterest.com
Infographic on Understanding FDA Device Classes from Medical Device Definition By Fda The information on this page is current as of mar 22, 2024. Fda regulates the sale of medical device products in the u.s. And monitors the safety of all regulated medical products. Overview of regulations for medical devices: The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Premarket notifications (510(k)), establishment. Medical Device Definition By Fda.
From mavink.com
Fda Medical Device Classification Chart Medical Device Definition By Fda Fda regulates the sale of medical device products in the u.s. An instrument, apparatus, implement, machine,. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Overview of regulations for medical devices: And monitors the safety of all regulated medical products. The information on this page is current as of mar 22,. Medical Device Definition By Fda.
From fyoqglxgv.blob.core.windows.net
What Is A Medical Device Fda Definition at Theresa Ward blog Medical Device Definition By Fda And monitors the safety of all regulated medical products. The information on this page is current as of mar 22, 2024. Fda regulates the sale of medical device products in the u.s. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: The food and drug administration (fda) has established classifications for approximately 1,700 different. Medical Device Definition By Fda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Device Definition By Fda And monitors the safety of all regulated medical products. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: An instrument, apparatus, implement, machine,. Exemptions from federal preemption of state and local medical device requirements: The information on this page is current as of mar 22, 2024. Fda regulates the sale of medical device products. Medical Device Definition By Fda.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Device Definition By Fda The information on this page is current as of mar 22, 2024. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Exemptions from federal preemption of state and local medical device requirements: And monitors the safety of all regulated medical products. The food and drug administration (fda) has established classifications for approximately 1,700 different. Medical Device Definition By Fda.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations Medical Device Definition By Fda An instrument, apparatus, implement, machine,. And monitors the safety of all regulated medical products. Overview of regulations for medical devices: Fda regulates the sale of medical device products in the u.s. Premarket notifications (510(k)), establishment registration, device listing,. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Exemptions from federal preemption of state and. Medical Device Definition By Fda.
From www.slideshare.net
US FDA medical device approval chart Emergo Medical Device Definition By Fda Overview of regulations for medical devices: Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: The information on this page is current as of mar 22, 2024. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Fda regulates the sale of medical device products in. Medical Device Definition By Fda.
From www.pinterest.com
FDA approach to Medical Device Classification Medical device, Medical Medical Device Definition By Fda The information on this page is current as of mar 22, 2024. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Fda regulates the sale of medical device products in the u.s. Overview of regulations for. Medical Device Definition By Fda.
From www.vrogue.co
What Is The Fda Definition Of A Medical Device Class vrogue.co Medical Device Definition By Fda The information on this page is current as of mar 22, 2024. Fda regulates the sale of medical device products in the u.s. And monitors the safety of all regulated medical products. An instrument, apparatus, implement, machine,. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Overview of regulations for medical devices: Exemptions from. Medical Device Definition By Fda.
From www.kolabtree.com
A guide to FDA Design Controls for your medical device Medical Device Definition By Fda Premarket notifications (510(k)), establishment registration, device listing,. Exemptions from federal preemption of state and local medical device requirements: And monitors the safety of all regulated medical products. The information on this page is current as of mar 22, 2024. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: The food and drug administration (fda). Medical Device Definition By Fda.
From www.slideserve.com
PPT The FDA Landscape AdvaMed September 2008 PowerPoint Presentation Medical Device Definition By Fda Premarket notifications (510(k)), establishment registration, device listing,. And monitors the safety of all regulated medical products. Overview of regulations for medical devices: An instrument, apparatus, implement, machine,. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as:. Medical Device Definition By Fda.
From www.greenlight.guru
FDA Cleared vs Approved vs Granted for Medical Devices Medical Device Definition By Fda Premarket notifications (510(k)), establishment registration, device listing,. The information on this page is current as of mar 22, 2024. An instrument, apparatus, implement, machine,. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. And monitors the safety of all regulated medical products. Overview of regulations for medical devices: Exemptions from federal. Medical Device Definition By Fda.