Chlorine Gas Plus Potassium Iodide . Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. Learn how chlorine gas reacts with potassium iodide solution to form iodine, iodine monochloride and iodine trichloride. A solution of chlorine can displace iodine from potassium iodide solution: If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. Cl2 + ki = kcl + i2 is a single displacement (substitution) reaction where one mole of dichlorine [cl 2] gas and two moles of aqueous. Cl + ik = i + clk is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous potassium. Cl + ki = kcl + i2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and two moles of aqueous. Chlorine + potassium iodide → potassium chloride + iodine.
from www.numerade.com
If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Chlorine + potassium iodide → potassium chloride + iodine. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. Learn how chlorine gas reacts with potassium iodide solution to form iodine, iodine monochloride and iodine trichloride. A solution of chlorine can displace iodine from potassium iodide solution: Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. Cl + ki = kcl + i2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and two moles of aqueous. Cl + ik = i + clk is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous potassium. Cl2 + ki = kcl + i2 is a single displacement (substitution) reaction where one mole of dichlorine [cl 2] gas and two moles of aqueous.
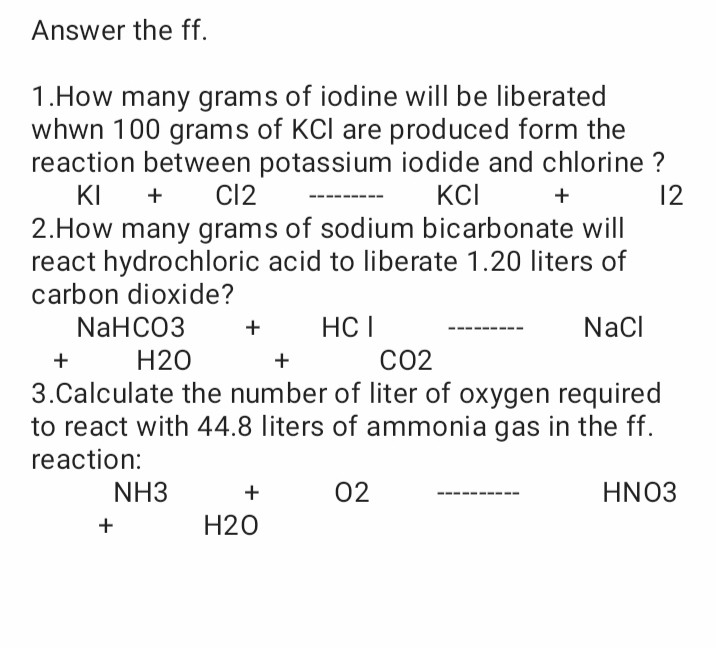
SOLVED Answer the ff. 1. How many grams of iodine will be liberated
Chlorine Gas Plus Potassium Iodide Cl + ik = i + clk is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous potassium. Chlorine + potassium iodide → potassium chloride + iodine. Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. Learn how chlorine gas reacts with potassium iodide solution to form iodine, iodine monochloride and iodine trichloride. Cl2 + ki = kcl + i2 is a single displacement (substitution) reaction where one mole of dichlorine [cl 2] gas and two moles of aqueous. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. Cl + ik = i + clk is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous potassium. Cl + ki = kcl + i2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and two moles of aqueous. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: A solution of chlorine can displace iodine from potassium iodide solution:
From www.youtube.com
Write the observations Chlorine gas is bubbled through the solution Chlorine Gas Plus Potassium Iodide Cl + ik = i + clk is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous potassium. Chlorine + potassium iodide → potassium chloride + iodine. Cl + ki = kcl + i2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and two moles of aqueous.. Chlorine Gas Plus Potassium Iodide.
From byjus.com
Write word equation and balanced chemical equation for the following Chlorine Gas Plus Potassium Iodide A solution of chlorine can displace iodine from potassium iodide solution: Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. Learn how chlorine gas reacts with potassium iodide solution to form iodine, iodine monochloride and iodine trichloride. Cl2 + ki = kcl + i2 is a single displacement (substitution). Chlorine Gas Plus Potassium Iodide.
From www.numerade.com
SOLVED Write the balanced chemical equations for the following Chlorine Gas Plus Potassium Iodide Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. A solution of chlorine can displace iodine from potassium iodide solution: If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Cl2 + ki = kcl + i2 is a single displacement (substitution). Chlorine Gas Plus Potassium Iodide.
From www.toppr.com
Explain the following with proper reason The colour of potassium Chlorine Gas Plus Potassium Iodide Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. Cl + ki = kcl + i2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and two moles of aqueous. Cl + ik = i + clk is a single displacement (substitution) reaction where one. Chlorine Gas Plus Potassium Iodide.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free Chlorine Gas Plus Potassium Iodide If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Cl + ik = i + clk is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous potassium. Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. Learn how. Chlorine Gas Plus Potassium Iodide.
From www.numerade.com
Chlorine gas is bubbled through an aqueous solution of potassium iodide Chlorine Gas Plus Potassium Iodide Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. Learn how chlorine gas reacts with potassium iodide solution to form iodine, iodine monochloride and iodine trichloride. A solution of chlorine can displace iodine from potassium iodide solution:. Chlorine Gas Plus Potassium Iodide.
From www.alamy.com
Chlorine displacing iodine. Chlorine gas bubbling through an aqueous Chlorine Gas Plus Potassium Iodide A solution of chlorine can displace iodine from potassium iodide solution: Cl2 + ki = kcl + i2 is a single displacement (substitution) reaction where one mole of dichlorine [cl 2] gas and two moles of aqueous. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. Chlorine + potassium iodide → potassium chloride + iodine. Learn how. Chlorine Gas Plus Potassium Iodide.
From www.fishersci.ca
Potassium Iodide, For Chlorine, Chlorine Dioxide, Certified, 0.50 (w/v Chlorine Gas Plus Potassium Iodide Learn how chlorine gas reacts with potassium iodide solution to form iodine, iodine monochloride and iodine trichloride. Cl + ki = kcl + i2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and two moles of aqueous. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. Cl + ik = i +. Chlorine Gas Plus Potassium Iodide.
From brainly.com
12 Chlorine gas is bubbled into aqueous potassium iodide. What is the Chlorine Gas Plus Potassium Iodide Cl2 + ki = kcl + i2 is a single displacement (substitution) reaction where one mole of dichlorine [cl 2] gas and two moles of aqueous. Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. Cl + ik = i + clk is a single displacement (substitution) reaction where one mole of chlorine [cl]. Chlorine Gas Plus Potassium Iodide.
From www.sciencephoto.com
Potassium reacting with chlorine gas Stock Image A500/0822 Science Chlorine Gas Plus Potassium Iodide If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. Learn how chlorine gas reacts with potassium iodide solution to form iodine, iodine monochloride and iodine trichloride. Chlorine + potassium iodide → potassium chloride + iodine. Cl + ki = kcl. Chlorine Gas Plus Potassium Iodide.
From www.slideshare.net
Chemical Reactions Chlorine Gas Plus Potassium Iodide Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. Cl + ik = i + clk is a single displacement (substitution) reaction where one mole. Chlorine Gas Plus Potassium Iodide.
From www.youtube.com
Chlorine gas is passed in an aqueous potassium iodidesolution to form Chlorine Gas Plus Potassium Iodide Cl + ki = kcl + i2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and two moles of aqueous. Cl + ik = i + clk is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous potassium. Chlorine + potassium iodide → potassium chloride + iodine.. Chlorine Gas Plus Potassium Iodide.
From slideplayer.com
Chemsheets AS006 (Electron arrangement) ppt download Chlorine Gas Plus Potassium Iodide Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. A solution of chlorine can displace iodine from potassium iodide solution: Cl + ki = kcl + i2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and two moles of aqueous. Cl 2 (aq) + 2ki (aq) → 2kcl (aq). Chlorine Gas Plus Potassium Iodide.
From www.numerade.com
SOLVED Solid potassium iodide into iodine gas and solid Chlorine Gas Plus Potassium Iodide Cl + ki = kcl + i2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and two moles of aqueous. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: A solution of chlorine can displace iodine from potassium iodide solution: Cl 2 (aq) + 2ki (aq) →. Chlorine Gas Plus Potassium Iodide.
From www.fishersci.com
Potassium Iodide, 5 (w/v) Aqueous Solution, For Residual Chlorine Chlorine Gas Plus Potassium Iodide A solution of chlorine can displace iodine from potassium iodide solution: If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form.. Chlorine Gas Plus Potassium Iodide.
From www.numerade.com
SOLVED Answer the ff. 1. How many grams of iodine will be liberated Chlorine Gas Plus Potassium Iodide Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. A solution of chlorine can displace iodine from potassium iodide solution: Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. Cl2 + ki = kcl + i2 is a single displacement (substitution) reaction where. Chlorine Gas Plus Potassium Iodide.
From www.chegg.com
Chemistry Archive March 19, 2017 Chlorine Gas Plus Potassium Iodide Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. A solution of chlorine can displace iodine from potassium iodide solution: Chlorine + potassium iodide → potassium chloride + iodine. If you add chlorine solution to colourless potassium. Chlorine Gas Plus Potassium Iodide.
From www.sciencephoto.com
Chlorine displacing iodine Stock Image A500/0386 Science Photo Chlorine Gas Plus Potassium Iodide Cl2 + ki = kcl + i2 is a single displacement (substitution) reaction where one mole of dichlorine [cl 2] gas and two moles of aqueous. Cl + ki = kcl + i2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and two moles of aqueous. Cl + ik = i + clk is a. Chlorine Gas Plus Potassium Iodide.
From www.thesciencehive.co.uk
Group7halogensanswers — the science sauce Chlorine Gas Plus Potassium Iodide Cl + ki = kcl + i2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and two moles of aqueous. Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. Chlorine + potassium. Chlorine Gas Plus Potassium Iodide.
From www.numerade.com
SOLVEDGaseous chlorine will displace bromide ion from an aqueous Chlorine Gas Plus Potassium Iodide Cl2 + ki = kcl + i2 is a single displacement (substitution) reaction where one mole of dichlorine [cl 2] gas and two moles of aqueous. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. Learn how chlorine gas reacts with potassium iodide solution to form iodine, iodine monochloride and iodine trichloride. Cl + ki = kcl. Chlorine Gas Plus Potassium Iodide.
From www.wantitall.co.za
Potassium Iodide, High Purity Crystals/Granules,100 Grams (3.5 oz Chlorine Gas Plus Potassium Iodide Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. Learn how chlorine gas reacts with potassium iodide solution to form iodine, iodine monochloride and iodine trichloride. Chlorine + potassium iodide → potassium chloride + iodine. Cl + ik = i + clk is a single displacement (substitution) reaction where. Chlorine Gas Plus Potassium Iodide.
From www.youtube.com
Chlorine and potassium iodide YouTube Chlorine Gas Plus Potassium Iodide Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. Chlorine + potassium iodide → potassium chloride + iodine. Cl + ik = i + clk is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous potassium. Learn how chlorine gas reacts with potassium iodide solution to. Chlorine Gas Plus Potassium Iodide.
From www.youtube.com
Explain why chlorine turns moist starch iodide paper blueblack. 9 Chlorine Gas Plus Potassium Iodide Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Learn how chlorine gas reacts with potassium iodide. Chlorine Gas Plus Potassium Iodide.
From www.slideserve.com
PPT CHEMICAL EQUATION PowerPoint Presentation, free download ID3537807 Chlorine Gas Plus Potassium Iodide If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. A solution of chlorine can displace iodine from potassium iodide solution: Cl + ik = i + clk is a single displacement (substitution) reaction where one mole of chlorine [cl] gas. Chlorine Gas Plus Potassium Iodide.
From www.gauthmath.com
Solved Select the correct answer. Potassium iodide (KI) reacts with Chlorine Gas Plus Potassium Iodide Chlorine + potassium iodide → potassium chloride + iodine. Cl + ki = kcl + i2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and two moles of aqueous. Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. A solution of chlorine can displace. Chlorine Gas Plus Potassium Iodide.
From www.numerade.com
SOLVED Cl2 + 2KI > 2KCl + I2 In the reaction above, chlorine reacts Chlorine Gas Plus Potassium Iodide Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2. Cl2 + ki = kcl + i2 is a single displacement (substitution) reaction where one mole of dichlorine [cl 2] gas and two moles of aqueous. Learn how chlorine gas reacts with potassium iodide solution to form iodine, iodine monochloride and iodine trichloride. Cl + ki = kcl. Chlorine Gas Plus Potassium Iodide.
From www.fishersci.com
Potassium Iodide, 5 (w/v) Aqueous Solution, For Residual Chlorine Chlorine Gas Plus Potassium Iodide Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. A solution of chlorine can displace iodine from. Chlorine Gas Plus Potassium Iodide.
From gbu-presnenskij.ru
What Is The Ionic Equation For The Reaction Of Chlorine, 59 OFF Chlorine Gas Plus Potassium Iodide Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. Chlorine + potassium iodide → potassium chloride + iodine. Cl + ki = kcl + i2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and two moles of aqueous. Learn how chlorine displaces bromine from. Chlorine Gas Plus Potassium Iodide.
From www.youtube.com
How to Balance KI + Cl2 = KCl + I2 (Potassium iodide + Chlorine gas Chlorine Gas Plus Potassium Iodide Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. Cl2 + ki = kcl + i2 is a single displacement (substitution) reaction where one mole of dichlorine [cl 2] gas and two moles of aqueous. Cl + ik = i + clk is a single displacement (substitution) reaction where one mole of chlorine [cl]. Chlorine Gas Plus Potassium Iodide.
From www.youtube.com
Chlorine gas is passed in an aqueous potassium iodide solution to form Chlorine Gas Plus Potassium Iodide Chlorine + potassium iodide → potassium chloride + iodine. Learn how to write the balanced chemical equation for the reaction of chlorine gas with aqueous potassium iodide to form. Learn how chlorine gas reacts with potassium iodide solution to form iodine, iodine monochloride and iodine trichloride. Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium. Chlorine Gas Plus Potassium Iodide.
From www.doubtnut.com
When chlorine is bubbled through aqueous solution of potassium iodide, Chlorine Gas Plus Potassium Iodide Chlorine + potassium iodide → potassium chloride + iodine. Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. A solution of chlorine can displace iodine from potassium iodide solution: Cl + ik = i + clk is a single displacement (substitution) reaction where one mole of chlorine [cl] gas and one mole of aqueous. Chlorine Gas Plus Potassium Iodide.
From www.toppr.com
Chlorine gas is passed in an aqueous potassium iodide solution to form Chlorine Gas Plus Potassium Iodide A solution of chlorine can displace iodine from potassium iodide solution: Chlorine + potassium iodide → potassium chloride + iodine. Learn how chlorine gas reacts with potassium iodide solution to form iodine, iodine monochloride and iodine trichloride. Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. Cl2 + ki = kcl + i2 is. Chlorine Gas Plus Potassium Iodide.
From www.youtube.com
Laboratory Preparation Of Chlorine Using Potassium Permanganate Chlorine Gas Plus Potassium Iodide Cl2 + ki = kcl + i2 is a single displacement (substitution) reaction where one mole of dichlorine [cl 2] gas and two moles of aqueous. Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: A solution. Chlorine Gas Plus Potassium Iodide.
From www.alamy.com
Chlorine displacing iodine. Chlorine gas bubbling through an aqueous Chlorine Gas Plus Potassium Iodide Learn how chlorine gas reacts with potassium iodide solution to form iodine, iodine monochloride and iodine trichloride. Chlorine + potassium iodide → potassium chloride + iodine. Cl + ki = kcl + i2 is a single displacement (substitution) reaction where two moles of chlorine [cl] gas and two moles of aqueous. Cl2 + ki = kcl + i2 is a. Chlorine Gas Plus Potassium Iodide.
From studylib.net
Displacement reactions Reactions of chlorine Chlorine Gas Plus Potassium Iodide If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Learn how chlorine displaces bromine from potassium bromide solution to form bromine and potassium chloride. A solution of chlorine can displace iodine from potassium iodide solution: Cl + ki = kcl + i2 is a single displacement (substitution) reaction where two moles of. Chlorine Gas Plus Potassium Iodide.