Copper Valence Electrons Number . To find the number of valence electrons for copper (cu)we need to look at its electron. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. To solve without a periodic table, find the electron configuration of the element and count the. Here, the electron configuration of copper ion (cu 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. The ones digit in the group number is the number of valence electrons. Yes, copper only has 1 valence electron. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. But for most of the transition. Valence electrons only include the electrons in the highest energy (n). This electron configuration shows that the copper ion has three shells and. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that.
from valenceelectrons.com
Yes, copper only has 1 valence electron. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. To solve without a periodic table, find the electron configuration of the element and count the. Here, the electron configuration of copper ion (cu 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The ones digit in the group number is the number of valence electrons. This electron configuration shows that the copper ion has three shells and. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Valence electrons only include the electrons in the highest energy (n). But for most of the transition.
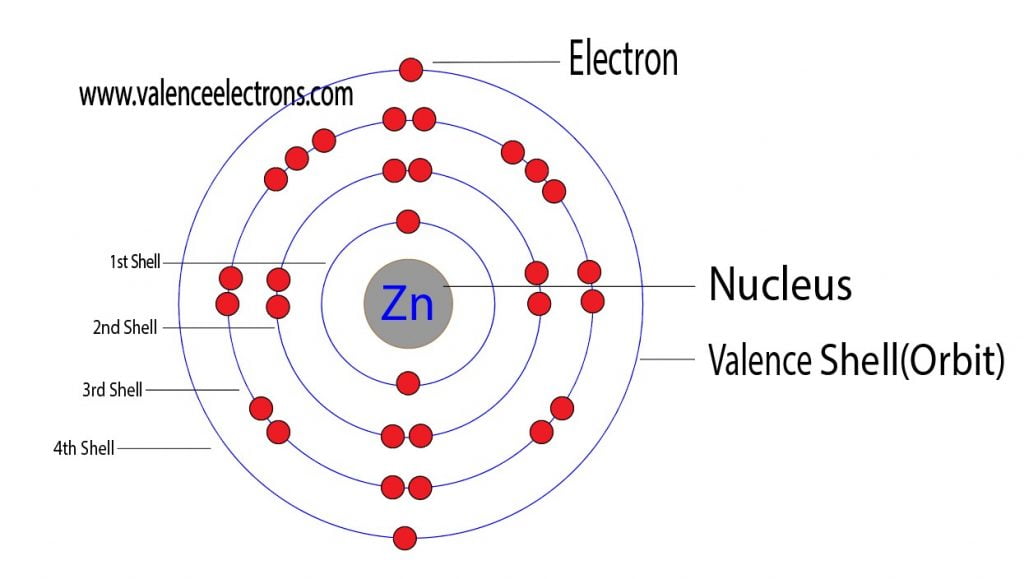
How Many Valence Electrons Does Zinc (Zn) Have?
Copper Valence Electrons Number Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. Here, the electron configuration of copper ion (cu 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. To solve without a periodic table, find the electron configuration of the element and count the. The ones digit in the group number is the number of valence electrons. But for most of the transition. Yes, copper only has 1 valence electron. This electron configuration shows that the copper ion has three shells and. Valence electrons only include the electrons in the highest energy (n). 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. To find the number of valence electrons for copper (cu)we need to look at its electron. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the.
From ar.inspiredpencil.com
How Many Valence Electrons Does Copper Have Copper Valence Electrons Number The ones digit in the group number is the number of valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Yes, copper only has 1 valence electron. To find the number of valence electrons for copper (cu)we need to look at its electron.. Copper Valence Electrons Number.
From valenceelectrons.com
How Many Valence Electrons Does Krypton (Kr) Have? Copper Valence Electrons Number To solve without a periodic table, find the electron configuration of the element and count the. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. To find the number of valence electrons for copper (cu)we need to look at its electron. Here, the. Copper Valence Electrons Number.
From strongnewsupdates.s3.amazonaws.com
Copper Valence Electrons Copper Valence Electrons Number Valence electrons only include the electrons in the highest energy (n). Here, the electron configuration of copper ion (cu 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. To find the number of valence electrons for copper (cu)we need to look at its electron. To solve without a periodic table, find the electron configuration of. Copper Valence Electrons Number.
From reviewhomedecor.co
Copper Periodic Table Electrons Review Home Decor Copper Valence Electrons Number Yes, copper only has 1 valence electron. The ones digit in the group number is the number of valence electrons. To solve without a periodic table, find the electron configuration of the element and count the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that.. Copper Valence Electrons Number.
From valenceelectrons.com
How Many Valence Electrons Does Silver (Ag) Have? Copper Valence Electrons Number This electron configuration shows that the copper ion has three shells and. The ones digit in the group number is the number of valence electrons. Here, the electron configuration of copper ion (cu 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. To solve without a periodic table, find the electron configuration of the element. Copper Valence Electrons Number.
From valenceelectrons.com
Protons, Neutrons, Electrons for (Sg, Sg6+) Copper Valence Electrons Number To solve without a periodic table, find the electron configuration of the element and count the. Valence electrons only include the electrons in the highest energy (n). This electron configuration shows that the copper ion has three shells and. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible. Copper Valence Electrons Number.
From ar.inspiredpencil.com
Copper Orbital Diagram Copper Valence Electrons Number To find the number of valence electrons for copper (cu)we need to look at its electron. Here, the electron configuration of copper ion (cu 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. The ones digit in the group number is the number of valence electrons. Valence electrons are the electrons that reside in the. Copper Valence Electrons Number.
From phootoscelebrities.com
How Many Valence Electrons Does Aluminum Have? Copper Valence Electrons Number Yes, copper only has 1 valence electron. The ones digit in the group number is the number of valence electrons. To find the number of valence electrons for copper (cu)we need to look at its electron. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation. Copper Valence Electrons Number.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Copper Valence Electrons Number But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Here, the electron configuration of copper ion (cu 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. Valence electrons only include the electrons in the. Copper Valence Electrons Number.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Valence Electrons Number This electron configuration shows that the copper ion has three shells and. Yes, copper only has 1 valence electron. To find the number of valence electrons for copper (cu)we need to look at its electron. The ones digit in the group number is the number of valence electrons. 93 rows you may assume the valences of the chemical elements—the number. Copper Valence Electrons Number.
From ar.inspiredpencil.com
How Many Valence Electrons Does Copper Have Copper Valence Electrons Number But for most of the transition. Here, the electron configuration of copper ion (cu 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. Yes, copper only has 1 valence electron. This electron configuration shows that the copper ion has three shells and. How to write the electron configuration for copper (cu, cu+, and cu2+) in. Copper Valence Electrons Number.
From www.expii.com
Valence Electrons — Definition & Importance Expii Copper Valence Electrons Number To solve without a periodic table, find the electron configuration of the element and count the. This electron configuration shows that the copper ion has three shells and. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The ones digit in the group number is. Copper Valence Electrons Number.
From reviewhomedecor.co
Copper Periodic Table Electrons Review Home Decor Copper Valence Electrons Number To solve without a periodic table, find the electron configuration of the element and count the. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an. Copper Valence Electrons Number.
From www.alamy.com
Symbol and electron diagram for Copper illustration Stock Vector Image Copper Valence Electrons Number To find the number of valence electrons for copper (cu)we need to look at its electron. Here, the electron configuration of copper ion (cu 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. Copper Valence Electrons Number.
From valenceelectrons.com
How Many Valence Electrons Does ClO2 and ClO2 Have? Copper Valence Electrons Number This electron configuration shows that the copper ion has three shells and. Valence electrons only include the electrons in the highest energy (n). Yes, copper only has 1 valence electron. To find the number of valence electrons for copper (cu)we need to look at its electron. Here, the electron configuration of copper ion (cu 2+) is 1s 2 2s 2. Copper Valence Electrons Number.
From pediaa.com
Difference Between Valency and Valence Electrons Copper Valence Electrons Number Yes, copper only has 1 valence electron. To find the number of valence electrons for copper (cu)we need to look at its electron. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. But for most of the transition. 93 rows you may. Copper Valence Electrons Number.
From www.scienceabc.com
What Are Valence Electrons And How To Find Them? Where Are They Located? Copper Valence Electrons Number But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. To find the number. Copper Valence Electrons Number.
From woodscribdindi.blogspot.com
valency table Scribd india Copper Valence Electrons Number Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. Valence electrons only include the electrons in the highest energy (n). To find the number of valence electrons for copper (cu)we need to look at its electron. 93 rows you may assume the. Copper Valence Electrons Number.
From www.wou.edu
Ch105 Chapter 2 Atoms, Elements and The Periodic Table Chemistry Copper Valence Electrons Number But for most of the transition. To solve without a periodic table, find the electron configuration of the element and count the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. To find the number of valence electrons for copper (cu)we need to look at. Copper Valence Electrons Number.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Copper Valence Electrons Number Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. Valence electrons only include the electrons in the highest energy (n). But for most of the transition. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write. Copper Valence Electrons Number.
From awesomehome.co
Copper Periodic Table Protons Neutrons And Electrons Awesome Home Copper Valence Electrons Number Here, the electron configuration of copper ion (cu 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. This electron configuration shows that the copper ion has three shells and. Valence electrons only include the electrons in the highest energy (n). Yes, copper only has 1 valence electron. To solve without a periodic table, find the. Copper Valence Electrons Number.
From mungfali.com
Periodic Table With Valence Electron Numbers Copper Valence Electrons Number The ones digit in the group number is the number of valence electrons. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Yes, copper only has 1 valence electron. But for most of the transition. This electron configuration shows that the copper ion. Copper Valence Electrons Number.
From www.youtube.com
How to Find the Valence Electrons for Copper (Cu) YouTube Copper Valence Electrons Number Here, the electron configuration of copper ion (cu 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. Yes, copper only has 1 valence electron. But for most of the transition. The ones digit in the group number is the number of valence electrons. 93 rows you may assume the valences of the chemical elements—the number. Copper Valence Electrons Number.
From www.technocrazed.com
2.3 Valence and Crystal Structure Copper Valence Electrons Number The ones digit in the group number is the number of valence electrons. To find the number of valence electrons for copper (cu)we need to look at its electron. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. Yes, copper only has. Copper Valence Electrons Number.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Valence Electrons Number Valence electrons only include the electrons in the highest energy (n). How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The ones digit in the group number is the number of valence electrons. This electron configuration shows that the copper ion has three. Copper Valence Electrons Number.
From printablekrafturjx.z21.web.core.windows.net
How To Identify The Valence Electron Copper Valence Electrons Number Here, the electron configuration of copper ion (cu 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. Valence electrons only include the electrons in the highest energy (n). Yes, copper only has 1 valence electron. This electron configuration shows that the copper ion has three shells and. But for most of the transition. 93 rows. Copper Valence Electrons Number.
From ar.inspiredpencil.com
How Many Valence Electrons Does Copper Have Copper Valence Electrons Number But for most of the transition. This electron configuration shows that the copper ion has three shells and. Yes, copper only has 1 valence electron. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The ones digit in the group number is. Copper Valence Electrons Number.
From valenceelectrons.com
How to Find the Valence Electrons for Copper (Cu)? Copper Valence Electrons Number Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. This electron configuration shows that the copper ion has three shells and. Valence electrons only include the electrons in the highest energy (n). How to write the electron configuration for copper (cu, cu+,. Copper Valence Electrons Number.
From valenceelectrons.com
How Many Valence Electrons Does Zinc (Zn) Have? Copper Valence Electrons Number How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. To solve without a periodic table, find the electron configuration of the element and count the. To find the number of valence electrons for copper (cu)we need to look at its electron. Valence electrons. Copper Valence Electrons Number.
From utedzz.blogspot.com
Periodic Table Of Elements Valence Electrons Periodic Table Timeline Copper Valence Electrons Number How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Valence electrons only include the electrons in the highest energy (n). To find the number of valence electrons for copper (cu)we need to look at its electron. Valence electrons are the electrons that reside. Copper Valence Electrons Number.
From reviewhomedecor.co
Copper Periodic Table Electrons Review Home Decor Copper Valence Electrons Number Here, the electron configuration of copper ion (cu 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. The ones digit in the group number is the number of valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Valence. Copper Valence Electrons Number.
From www.youtube.com
How to Find the Valence Electrons for Copper II Easy & Quick YouTube Copper Valence Electrons Number Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. To solve without a periodic table, find the electron configuration of the element and count the. The ones digit in the group number is the number of valence electrons. How to write the. Copper Valence Electrons Number.