Evaporation Temperature Of Mercury . Data on the isotopes of mercury, melting point, boiling point,. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. The atomic and physical properties of elemental mercury are covered. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Mercury has a relatively high vapor pressure, even at room temperature. To understand that the relationship between pressure,.
from www.motherjones.com
To understand that the relationship between pressure,. The atomic and physical properties of elemental mercury are covered. Data on the isotopes of mercury, melting point, boiling point,. Mercury has a relatively high vapor pressure, even at room temperature. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present.
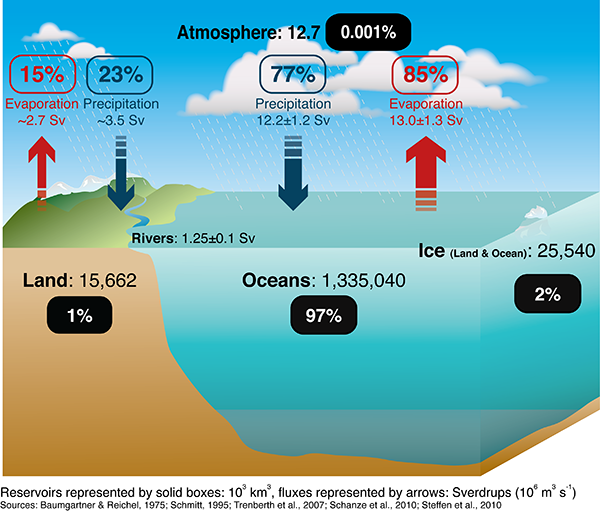
Big Changes in Ocean Salinity Intensifying Water Cycle Mother Jones
Evaporation Temperature Of Mercury To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. The atomic and physical properties of elemental mercury are covered. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. To understand that the relationship between pressure,. Mercury has a relatively high vapor pressure, even at room temperature. Data on the isotopes of mercury, melting point, boiling point,.
From vacaero.com
Evaporation Evaporation Temperature Of Mercury The atomic and physical properties of elemental mercury are covered. To understand that the relationship between pressure,. Data on the isotopes of mercury, melting point, boiling point,. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is. Evaporation Temperature Of Mercury.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Evaporation Temperature Of Mercury To understand that the relationship between pressure,. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. Data on the isotopes of mercury, melting point, boiling point,. Mercury has. Evaporation Temperature Of Mercury.
From www.facebook.com
Facebook Evaporation Temperature Of Mercury The atomic and physical properties of elemental mercury are covered. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. Mercury has a relatively high vapor pressure, even at room temperature. To understand that the relationship between pressure,. To understand that the equilibrium vapor pressure of a liquid. Evaporation Temperature Of Mercury.
From www.numerade.com
SOLVED What is the primary for more than rrcason why liquid mercury Evaporation Temperature Of Mercury To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. To understand that the relationship between pressure,. Data on the isotopes of mercury, melting point, boiling point,. Mercury has a relatively high vapor pressure, even at room temperature. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which. Evaporation Temperature Of Mercury.
From www.mdpi.com
Vapor Pressure versus Temperature Relations of Common Elements Evaporation Temperature Of Mercury For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. To understand that the relationship between pressure,. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Mercury has a relatively high. Evaporation Temperature Of Mercury.
From www.researchgate.net
The evaporation levels at different solution preparation temperatures Evaporation Temperature Of Mercury To understand that the relationship between pressure,. Mercury has a relatively high vapor pressure, even at room temperature. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. The atomic and physical properties of elemental mercury are covered. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which. Evaporation Temperature Of Mercury.
From www.indium.com
Properties of Gallium & Gallium Alloys Indium Corporation Evaporation Temperature Of Mercury To understand that the relationship between pressure,. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. The input energy required to. Evaporation Temperature Of Mercury.
From cupsoguepictures.com
️ Heat of vaporization of water lab. Vapor Pressure and Heat Evaporation Temperature Of Mercury The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Data on the isotopes of mercury, melting point, boiling point,. The atomic and physical properties of elemental mercury are. Evaporation Temperature Of Mercury.
From www.tessshebaylo.com
Evaporation Rate Equation Vapor Pressure Tessshebaylo Evaporation Temperature Of Mercury For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. Data on the isotopes of mercury, melting point, boiling point,. To understand that the relationship between pressure,. To understand that the equilibrium vapor pressure of a liquid depends on the temperature. Evaporation Temperature Of Mercury.
From www.mdpi.com
Energies Free FullText Simulation Study on the Performance of an Evaporation Temperature Of Mercury Mercury has a relatively high vapor pressure, even at room temperature. The atomic and physical properties of elemental mercury are covered. To understand that the relationship between pressure,. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. For every 10°c increase in temperature, the evaporation rate of. Evaporation Temperature Of Mercury.
From www.researchgate.net
The temperatures of the outlet cooling water at different evaporation Evaporation Temperature Of Mercury To understand that the relationship between pressure,. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. The atomic. Evaporation Temperature Of Mercury.
From blogdejuanmateacher.blogspot.com
El blog de juanmateacher 6º CHANGES OF STATE OF MATTER Evaporation Temperature Of Mercury For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. The atomic and physical properties of elemental mercury are. Evaporation Temperature Of Mercury.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation Evaporation Temperature Of Mercury The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Mercury has a relatively high vapor pressure, even at room temperature. The atomic and physical properties of elemental mercury. Evaporation Temperature Of Mercury.
From www.carid.com
Mercury OE 8L8Z19E628AA A/C Evaporator Temperature Sensor Evaporation Temperature Of Mercury To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. The atomic and physical properties of elemental mercury are covered. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. The input. Evaporation Temperature Of Mercury.
From www.alamy.com
Mercury in glass thermometer hires stock photography and images Alamy Evaporation Temperature Of Mercury To understand that the relationship between pressure,. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. Data on the isotopes of mercury, melting point, boiling point,. The atomic and physical properties of elemental mercury are covered. To understand that the. Evaporation Temperature Of Mercury.
From www.researchgate.net
Change of COP in various evaporation temperatures for different Evaporation Temperature Of Mercury For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Mercury has a relatively high vapor pressure, even at room temperature. The. Evaporation Temperature Of Mercury.
From www.numerade.com
SOLVED A vapour absorption refrigeration system works with a generator Evaporation Temperature Of Mercury The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. To understand that the relationship between pressure,. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. To understand. Evaporation Temperature Of Mercury.
From www.researchgate.net
(PDF) Evaporation of mercury at dropped pressure Evaporation Temperature Of Mercury Data on the isotopes of mercury, melting point, boiling point,. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. Mercury has a relatively high vapor pressure, even at room temperature. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires. Evaporation Temperature Of Mercury.
From www.powerstream.com
Vapor pressures of the Chemical Elements, vapor pressure of metals and Evaporation Temperature Of Mercury To understand that the relationship between pressure,. The atomic and physical properties of elemental mercury are covered. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. For every. Evaporation Temperature Of Mercury.
From saylordotorg.github.io
Properties of Liquids Evaporation Temperature Of Mercury To understand that the relationship between pressure,. The atomic and physical properties of elemental mercury are covered. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. Mercury has. Evaporation Temperature Of Mercury.
From www.justanswer.com
I need to bypass the evaporator air temperature sensor in a 2010 Ford Evaporation Temperature Of Mercury For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. The atomic and physical properties of elemental mercury are covered. Data on. Evaporation Temperature Of Mercury.
From www.researchgate.net
The effect of generator and evaporator temperatures on the COP Evaporation Temperature Of Mercury Data on the isotopes of mercury, melting point, boiling point,. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. To understand that the relationship between pressure,. The input energy required to change the state from liquid to vapor at constant. Evaporation Temperature Of Mercury.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Evaporation Temperature Of Mercury For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. The atomic and physical properties of elemental mercury are. Evaporation Temperature Of Mercury.
From www.planetary.org
Temperatures at Mercury's south pole The Society Evaporation Temperature Of Mercury Mercury has a relatively high vapor pressure, even at room temperature. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. To. Evaporation Temperature Of Mercury.
From sensordiary.com
How To Test Evaporator Temperature Sensor? By Sensor Diary Sensor Diary Evaporation Temperature Of Mercury For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. The atomic and physical properties of elemental mercury are covered. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of. Evaporation Temperature Of Mercury.
From www.motherjones.com
Big Changes in Ocean Salinity Intensifying Water Cycle Mother Jones Evaporation Temperature Of Mercury To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. The input energy required to change the state from liquid to vapor. Evaporation Temperature Of Mercury.
From www.studocu.com
Chapter 02 An ammonia vapor refrigeration cycle operates at an Evaporation Temperature Of Mercury For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. To understand that the relationship between pressure,. Data on. Evaporation Temperature Of Mercury.
From www.vrogue.co
Considering The Temperature Vs Time Graph Below How D vrogue.co Evaporation Temperature Of Mercury For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. To understand that the relationship between pressure,. Data on the isotopes of mercury, melting point, boiling point,. To understand that the equilibrium vapor pressure of a liquid depends on the temperature. Evaporation Temperature Of Mercury.
From revivalportal.goodwood.com
Ford Evaporator Temperature Sensor Evaporation Temperature Of Mercury For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. To understand that the relationship between pressure,. Data on the isotopes of mercury, melting point, boiling point,. The atomic and physical properties of elemental mercury are covered. The input energy required. Evaporation Temperature Of Mercury.
From romunpress.co.nz
The Evaporation of Mercury Romun Nose Evaporation Temperature Of Mercury Mercury has a relatively high vapor pressure, even at room temperature. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. To understand that the relationship between pressure,. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. Data. Evaporation Temperature Of Mercury.
From saylordotorg.github.io
Vapor Pressure Evaporation Temperature Of Mercury For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Data on the isotopes of mercury, melting point, boiling point,. The atomic. Evaporation Temperature Of Mercury.
From www.animalia-life.club
Evaporates Evaporation Temperature Of Mercury The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. Mercury has a relatively high vapor pressure, even at room temperature. The atomic and physical properties of elemental mercury are covered. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular. Evaporation Temperature Of Mercury.
From www.researchgate.net
The relationship between PR and freshwater production with the Evaporation Temperature Of Mercury The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. The atomic and physical properties of elemental mercury are. Evaporation Temperature Of Mercury.
From www.researchgate.net
For two different evaporation temperatures the dependence of the Evaporation Temperature Of Mercury Mercury has a relatively high vapor pressure, even at room temperature. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. The. Evaporation Temperature Of Mercury.
From www.youtube.com
3.3 A Evaporation, temperature YouTube Evaporation Temperature Of Mercury The atomic and physical properties of elemental mercury are covered. To understand that the relationship between pressure,. Mercury has a relatively high vapor pressure, even at room temperature. For every 10°c increase in temperature, the evaporation rate of mercury doubles, which is why mercury fires often have very serious consequences due to the strong evaporation of mercury,. To understand that. Evaporation Temperature Of Mercury.