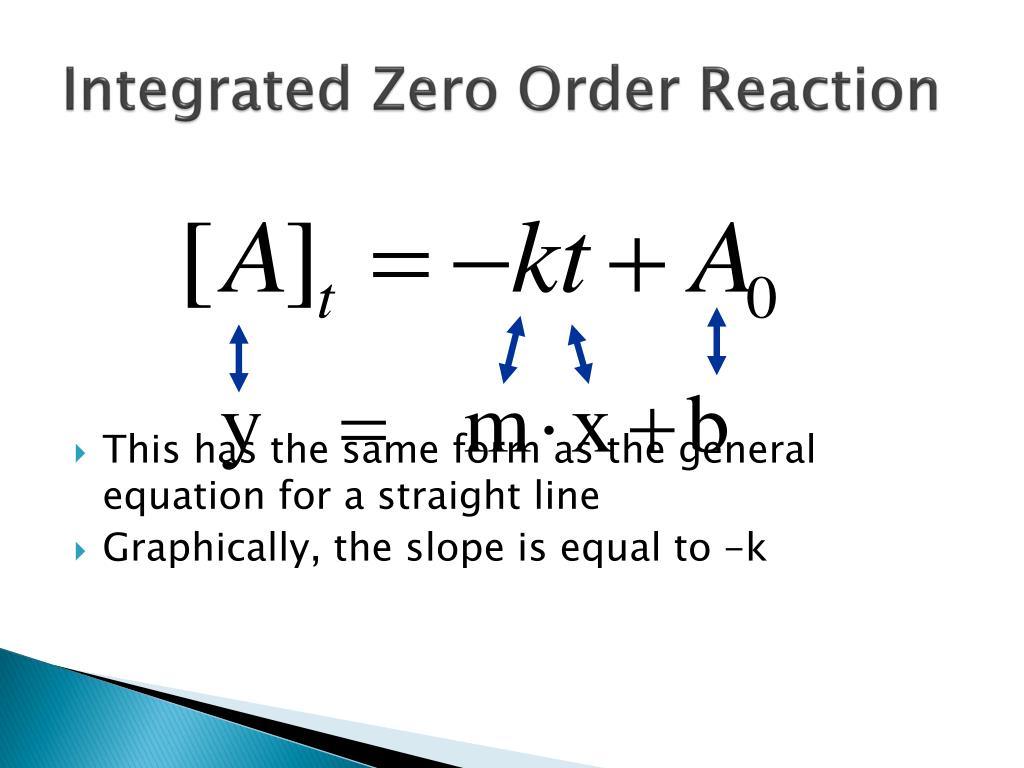
Half Life Equation For Zero Order Reaction . Plugging these in the equation for concentration, we get. For a first order reaction a products , rate = k [a]: T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: $$ \frac {1} {2} [a]_0 =−k t_. T ½ = 0.693 / k. For a second order reaction 2a products or a. Substituting into the integrated rate law: Concentration (\([a]\)) in a zero order reaction, several observation can be made: The slope of this plot is a straight line with negative slope equal. \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. Below is a graph of time (\(t\)) vs. => t 1/2 = [a] o /2k.
from www.slideserve.com
Concentration (\([a]\)) in a zero order reaction, several observation can be made: The slope of this plot is a straight line with negative slope equal. => t 1/2 = [a] o /2k. For a zero order reaction a products , rate = k: T ½ = [a o] / 2k. Substituting into the integrated rate law: T ½ = 0.693 / k. $$ \frac {1} {2} [a]_0 =−k t_. For a second order reaction 2a products or a. Below is a graph of time (\(t\)) vs.
PPT Chemical PowerPoint Presentation, free download ID2054453
Half Life Equation For Zero Order Reaction The slope of this plot is a straight line with negative slope equal. T ½ = [a o] / 2k. The slope of this plot is a straight line with negative slope equal. T ½ = 0.693 / k. $$ \frac {1} {2} [a]_0 =−k t_. For a zero order reaction a products , rate = k: Concentration (\([a]\)) in a zero order reaction, several observation can be made: => t 1/2 = [a] o /2k. For a first order reaction a products , rate = k [a]: Substituting into the integrated rate law: For a second order reaction 2a products or a. \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. Below is a graph of time (\(t\)) vs. Plugging these in the equation for concentration, we get.
From chemguru.sg
Rate Equation and Order of Reaction Half Life Equation For Zero Order Reaction => t 1/2 = [a] o /2k. The slope of this plot is a straight line with negative slope equal. For a second order reaction 2a products or a. T ½ = 0.693 / k. Substituting into the integrated rate law: Plugging these in the equation for concentration, we get. For a first order reaction a products , rate =. Half Life Equation For Zero Order Reaction.
From brainly.in
prove that half life period of zero order reaction is proportional to Half Life Equation For Zero Order Reaction Concentration (\([a]\)) in a zero order reaction, several observation can be made: The slope of this plot is a straight line with negative slope equal. $$ \frac {1} {2} [a]_0 =−k t_. Substituting into the integrated rate law: \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. T ½ = [a o] / 2k. Below. Half Life Equation For Zero Order Reaction.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID999616 Half Life Equation For Zero Order Reaction => t 1/2 = [a] o /2k. Substituting into the integrated rate law: For a second order reaction 2a products or a. Concentration (\([a]\)) in a zero order reaction, several observation can be made: $$ \frac {1} {2} [a]_0 =−k t_. T ½ = [a o] / 2k. T ½ = 0.693 / k. Below is a graph of time. Half Life Equation For Zero Order Reaction.
From lauranfryer.blogspot.com
half life formula for first order reaction Lauran Fryer Half Life Equation For Zero Order Reaction Plugging these in the equation for concentration, we get. Substituting into the integrated rate law: T ½ = [a o] / 2k. \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. For a zero order reaction a products , rate = k: For a second order reaction 2a products or a. Concentration (\([a]\)) in a. Half Life Equation For Zero Order Reaction.
From www.youtube.com
Half Life Equation Derivation YouTube Half Life Equation For Zero Order Reaction For a second order reaction 2a products or a. $$ \frac {1} {2} [a]_0 =−k t_. T ½ = 0.693 / k. Substituting into the integrated rate law: Concentration (\([a]\)) in a zero order reaction, several observation can be made: Plugging these in the equation for concentration, we get. The slope of this plot is a straight line with negative. Half Life Equation For Zero Order Reaction.
From louratilley.blogspot.com
half life formula for zero order reaction Loura Tilley Half Life Equation For Zero Order Reaction For a first order reaction a products , rate = k [a]: T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: For a second order reaction 2a products or a. => t 1/2 = [a] o /2k. Below is a graph of time (\(t\)) vs. T ½ = 0.693 / k.. Half Life Equation For Zero Order Reaction.
From lauranfryer.blogspot.com
half life formula for first order reaction Lauran Fryer Half Life Equation For Zero Order Reaction => t 1/2 = [a] o /2k. For a second order reaction 2a products or a. \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. T ½ = 0.693 / k. For a zero order reaction a products , rate = k: Substituting into the integrated rate law: The slope of this plot is a. Half Life Equation For Zero Order Reaction.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID2054453 Half Life Equation For Zero Order Reaction Concentration (\([a]\)) in a zero order reaction, several observation can be made: $$ \frac {1} {2} [a]_0 =−k t_. T ½ = [a o] / 2k. Plugging these in the equation for concentration, we get. Substituting into the integrated rate law: T ½ = 0.693 / k. Below is a graph of time (\(t\)) vs. => t 1/2 = [a]. Half Life Equation For Zero Order Reaction.
From scienceinfo.com
Zeroorder Reaction Rate Equation, Unit, Graph, Example Half Life Equation For Zero Order Reaction $$ \frac {1} {2} [a]_0 =−k t_. Plugging these in the equation for concentration, we get. Substituting into the integrated rate law: => t 1/2 = [a] o /2k. T ½ = [a o] / 2k. For a second order reaction 2a products or a. \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. For. Half Life Equation For Zero Order Reaction.
From byjus.com
Half life of a certain zero order reaction, A → P is 2 hour when the Half Life Equation For Zero Order Reaction Below is a graph of time (\(t\)) vs. T ½ = 0.693 / k. => t 1/2 = [a] o /2k. $$ \frac {1} {2} [a]_0 =−k t_. Plugging these in the equation for concentration, we get. For a second order reaction 2a products or a. \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed.. Half Life Equation For Zero Order Reaction.
From www.toppr.com
Derive the relationship between half life period and rate constant of a Half Life Equation For Zero Order Reaction The slope of this plot is a straight line with negative slope equal. For a first order reaction a products , rate = k [a]: Concentration (\([a]\)) in a zero order reaction, several observation can be made: => t 1/2 = [a] o /2k. \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. Below is. Half Life Equation For Zero Order Reaction.
From www.youtube.com
Determine the halflife of a zero order reaction YouTube Half Life Equation For Zero Order Reaction The slope of this plot is a straight line with negative slope equal. T ½ = 0.693 / k. For a zero order reaction a products , rate = k: Substituting into the integrated rate law: For a second order reaction 2a products or a. => t 1/2 = [a] o /2k. \[[a]=−kt+[a]_0 \nonumber \] when half of the initial. Half Life Equation For Zero Order Reaction.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free Half Life Equation For Zero Order Reaction $$ \frac {1} {2} [a]_0 =−k t_. Below is a graph of time (\(t\)) vs. For a first order reaction a products , rate = k [a]: The slope of this plot is a straight line with negative slope equal. T ½ = [a o] / 2k. Concentration (\([a]\)) in a zero order reaction, several observation can be made: =>. Half Life Equation For Zero Order Reaction.
From www.tessshebaylo.com
Derive Integrated Rate Equation For First Order Reaction Tessshebaylo Half Life Equation For Zero Order Reaction For a first order reaction a products , rate = k [a]: For a zero order reaction a products , rate = k: T ½ = 0.693 / k. Concentration (\([a]\)) in a zero order reaction, several observation can be made: T ½ = [a o] / 2k. $$ \frac {1} {2} [a]_0 =−k t_. Plugging these in the equation. Half Life Equation For Zero Order Reaction.
From edurev.in
Halflife of a zero order reaction is 250sec. t75 ,t100 of the Half Life Equation For Zero Order Reaction => t 1/2 = [a] o /2k. Plugging these in the equation for concentration, we get. The slope of this plot is a straight line with negative slope equal. \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. For a zero order reaction a products , rate = k: For a second order reaction 2a. Half Life Equation For Zero Order Reaction.
From www.youtube.com
half life of zero order reaction(chemical part 48 for CBSE Half Life Equation For Zero Order Reaction Below is a graph of time (\(t\)) vs. For a first order reaction a products , rate = k [a]: For a zero order reaction a products , rate = k: Plugging these in the equation for concentration, we get. T ½ = [a o] / 2k. The slope of this plot is a straight line with negative slope equal.. Half Life Equation For Zero Order Reaction.
From www.slideserve.com
PPT Chemical Chapter 14 PowerPoint Presentation, free Half Life Equation For Zero Order Reaction Concentration (\([a]\)) in a zero order reaction, several observation can be made: \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. T ½ = 0.693 / k. For a zero order reaction a products , rate = k: T ½ = [a o] / 2k. => t 1/2 = [a] o /2k. The slope of. Half Life Equation For Zero Order Reaction.
From www.youtube.com
Derivation of Half life period of Zero order reaction(chemical Half Life Equation For Zero Order Reaction $$ \frac {1} {2} [a]_0 =−k t_. For a second order reaction 2a products or a. T ½ = [a o] / 2k. => t 1/2 = [a] o /2k. Plugging these in the equation for concentration, we get. Concentration (\([a]\)) in a zero order reaction, several observation can be made: T ½ = 0.693 / k. For a zero. Half Life Equation For Zero Order Reaction.
From askfilo.com
Half Life of a Zero Order ReactionThe half life of a reaction is the tim.. Half Life Equation For Zero Order Reaction The slope of this plot is a straight line with negative slope equal. For a zero order reaction a products , rate = k: \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. T ½ = 0.693 / k. Below is a graph of time (\(t\)) vs. For a first order reaction a products ,. Half Life Equation For Zero Order Reaction.
From www.slideserve.com
PPT Chapter 12 Chemical PowerPoint Presentation, free Half Life Equation For Zero Order Reaction => t 1/2 = [a] o /2k. Substituting into the integrated rate law: Below is a graph of time (\(t\)) vs. \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. Plugging these in the equation for concentration, we get. $$ \frac {1} {2} [a]_0 =−k t_. For a zero order reaction a products , rate. Half Life Equation For Zero Order Reaction.
From www.slideserve.com
PPT Chapter 15 Chemical The Rates of Chemical Reactions Half Life Equation For Zero Order Reaction T ½ = [a o] / 2k. Substituting into the integrated rate law: For a zero order reaction a products , rate = k: Concentration (\([a]\)) in a zero order reaction, several observation can be made: Below is a graph of time (\(t\)) vs. The slope of this plot is a straight line with negative slope equal. T ½ =. Half Life Equation For Zero Order Reaction.
From www.youtube.com
Integrated Rate Laws Zero, First, & Second Order Reactions Chemical Half Life Equation For Zero Order Reaction => t 1/2 = [a] o /2k. Concentration (\([a]\)) in a zero order reaction, several observation can be made: Below is a graph of time (\(t\)) vs. For a second order reaction 2a products or a. For a zero order reaction a products , rate = k: \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been. Half Life Equation For Zero Order Reaction.
From www.toppr.com
Derive an integrated rate equation for rate constant of a zero order Half Life Equation For Zero Order Reaction For a second order reaction 2a products or a. For a first order reaction a products , rate = k [a]: Below is a graph of time (\(t\)) vs. => t 1/2 = [a] o /2k. The slope of this plot is a straight line with negative slope equal. T ½ = [a o] / 2k. For a zero order. Half Life Equation For Zero Order Reaction.
From louratilley.blogspot.com
half life formula for zero order reaction Loura Tilley Half Life Equation For Zero Order Reaction The slope of this plot is a straight line with negative slope equal. For a second order reaction 2a products or a. Concentration (\([a]\)) in a zero order reaction, several observation can be made: => t 1/2 = [a] o /2k. \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. T ½ = 0.693 /. Half Life Equation For Zero Order Reaction.
From www.youtube.com
Zeroth, First and Second Order Reactions YouTube Half Life Equation For Zero Order Reaction T ½ = 0.693 / k. Concentration (\([a]\)) in a zero order reaction, several observation can be made: Below is a graph of time (\(t\)) vs. => t 1/2 = [a] o /2k. $$ \frac {1} {2} [a]_0 =−k t_. For a second order reaction 2a products or a. For a zero order reaction a products , rate = k:. Half Life Equation For Zero Order Reaction.
From www.youtube.com
Integrated rate equation & half life of zero order reaction.(class 12 Half Life Equation For Zero Order Reaction \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. For a first order reaction a products , rate = k [a]: => t 1/2 = [a] o /2k. Plugging these in the equation for concentration, we get. Below is a graph of time (\(t\)) vs. For a second order reaction 2a products or a. Substituting. Half Life Equation For Zero Order Reaction.
From general.chemistrysteps.com
SecondOrder Reactions Chemistry Steps Half Life Equation For Zero Order Reaction For a zero order reaction a products , rate = k: T ½ = 0.693 / k. Plugging these in the equation for concentration, we get. Below is a graph of time (\(t\)) vs. => t 1/2 = [a] o /2k. The slope of this plot is a straight line with negative slope equal. Concentration (\([a]\)) in a zero order. Half Life Equation For Zero Order Reaction.
From general.chemistrysteps.com
SecondOrder Reactions Chemistry Steps Half Life Equation For Zero Order Reaction Substituting into the integrated rate law: \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. For a first order reaction a products , rate = k [a]: Below is a graph of time (\(t\)) vs. T ½ = [a o] / 2k. For a second order reaction 2a products or a. T ½ = 0.693. Half Life Equation For Zero Order Reaction.
From byjus.com
Half life for the zero order reaction A(g)→ B(g) +C(g) and half life Half Life Equation For Zero Order Reaction The slope of this plot is a straight line with negative slope equal. Plugging these in the equation for concentration, we get. $$ \frac {1} {2} [a]_0 =−k t_. For a zero order reaction a products , rate = k: T ½ = [a o] / 2k. Below is a graph of time (\(t\)) vs. => t 1/2 = [a]. Half Life Equation For Zero Order Reaction.
From byjus.com
Time taken to complete half life of zero order reaction is 75 secs Half Life Equation For Zero Order Reaction For a zero order reaction a products , rate = k: Plugging these in the equation for concentration, we get. For a first order reaction a products , rate = k [a]: Substituting into the integrated rate law: T ½ = [a o] / 2k. $$ \frac {1} {2} [a]_0 =−k t_. The slope of this plot is a straight. Half Life Equation For Zero Order Reaction.
From www.youtube.com
How to Calculate HalfLife for Second Order Reactions YouTube Half Life Equation For Zero Order Reaction Substituting into the integrated rate law: Below is a graph of time (\(t\)) vs. \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. For a first order reaction a products , rate = k [a]: For a second order reaction 2a products or a. The slope of this plot is a straight line with negative. Half Life Equation For Zero Order Reaction.
From www.slideserve.com
PPT Summary of the of ZeroOrder, FirstOrder and Second Half Life Equation For Zero Order Reaction Below is a graph of time (\(t\)) vs. For a zero order reaction a products , rate = k: \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. Plugging these in the equation for concentration, we get. T ½ = 0.693 / k. For a first order reaction a products , rate = k [a]:. Half Life Equation For Zero Order Reaction.
From www.coursehero.com
[Solved] Derive the integrated rate law and the halflife equations for Half Life Equation For Zero Order Reaction $$ \frac {1} {2} [a]_0 =−k t_. The slope of this plot is a straight line with negative slope equal. For a first order reaction a products , rate = k [a]: Below is a graph of time (\(t\)) vs. => t 1/2 = [a] o /2k. Concentration (\([a]\)) in a zero order reaction, several observation can be made: T. Half Life Equation For Zero Order Reaction.
From ar.inspiredpencil.com
Half Life Equation Algebra Half Life Equation For Zero Order Reaction Concentration (\([a]\)) in a zero order reaction, several observation can be made: T ½ = 0.693 / k. For a zero order reaction a products , rate = k: \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. For a second order reaction 2a products or a. $$ \frac {1} {2} [a]_0 =−k t_. =>. Half Life Equation For Zero Order Reaction.
From www.chemistrynotmystery.com
Zero order reaction and its half life Chemistry!!! Not Mystery Half Life Equation For Zero Order Reaction The slope of this plot is a straight line with negative slope equal. For a first order reaction a products , rate = k [a]: Plugging these in the equation for concentration, we get. T ½ = [a o] / 2k. \[[a]=−kt+[a]_0 \nonumber \] when half of the initial amount of reactant has been consumed. For a second order reaction. Half Life Equation For Zero Order Reaction.