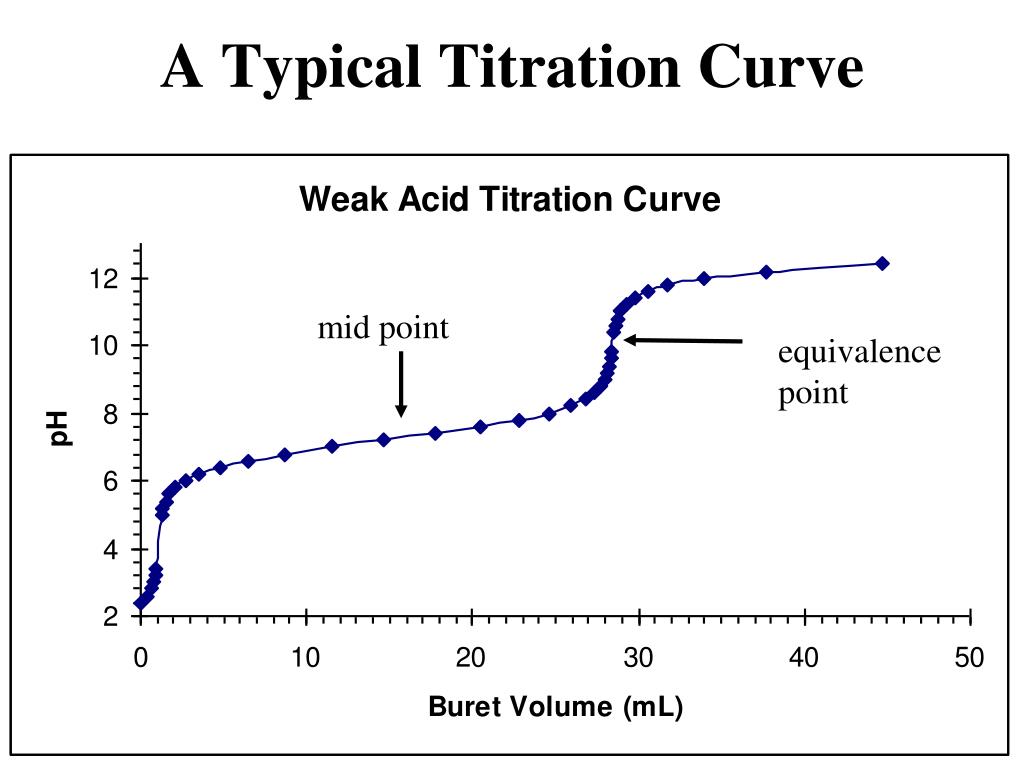
Titration Buffer Equation . ph = pk a + log [base / acid] is often the way you see it written on the internet. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). When a weak acid is titrated against a strong base, the ph of. Many q&a forums still lack the ability to make a. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). In chemistry, a buffer zone is a region where the ph of a solution remains constant.
from exoyzonai.blob.core.windows.net
In chemistry, a buffer zone is a region where the ph of a solution remains constant. When a weak acid is titrated against a strong base, the ph of. in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. ph = pk a + log [base / acid] is often the way you see it written on the internet. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). Many q&a forums still lack the ability to make a. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base.
Titration Curve Labeled Buffer Region at Craig Johnson blog
Titration Buffer Equation Many q&a forums still lack the ability to make a. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. In chemistry, a buffer zone is a region where the ph of a solution remains constant. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. When a weak acid is titrated against a strong base, the ph of. in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). ph = pk a + log [base / acid] is often the way you see it written on the internet. Many q&a forums still lack the ability to make a.
From www.youtube.com
Buffers and Titration Curves YouTube Titration Buffer Equation ph = pk a + log [base / acid] is often the way you see it written on the internet. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. for the titration of a weak base with a strong acid, the ph curve is initially basic. Titration Buffer Equation.
From fyoltrxao.blob.core.windows.net
The Titration Equation Is at Lana Tracy blog Titration Buffer Equation ph = pk a + log [base / acid] is often the way you see it written on the internet. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). Many q&a forums still lack the ability to make a. When a. Titration Buffer Equation.
From www.vrogue.co
How Do Buffers Work An Easy Explaination For Biologis vrogue.co Titration Buffer Equation In chemistry, a buffer zone is a region where the ph of a solution remains constant. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). Many q&a forums still lack the ability to make a. When a weak acid is titrated against. Titration Buffer Equation.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation Titration Buffer Equation In chemistry, a buffer zone is a region where the ph of a solution remains constant. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. for the titration of a weak base with a strong acid, the ph curve is. Titration Buffer Equation.
From www.slideshare.net
Tang 07 titrations Titration Buffer Equation ph = pk a + log [base / acid] is often the way you see it written on the internet. In chemistry, a buffer zone is a region where the ph of a solution remains constant. When a weak acid is titrated against a strong base, the ph of. in this section, we will see how to perform. Titration Buffer Equation.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Buffer Equation In chemistry, a buffer zone is a region where the ph of a solution remains constant. ph = pk a + log [base / acid] is often the way you see it written on the internet. in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). Many q&a. Titration Buffer Equation.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Buffer Equation In chemistry, a buffer zone is a region where the ph of a solution remains constant. ph = pk a + log [base / acid] is often the way you see it written on the internet. Many q&a forums still lack the ability to make a. in a buffer solution, the weak acid (or the weak base) is. Titration Buffer Equation.
From socratic.org
Titration curve? Please help... Socratic Titration Buffer Equation in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). In chemistry, a buffer zone is a region where the ph of a solution remains constant. When a weak acid is titrated against a strong base, the ph of. in this section, we will see how to perform. Titration Buffer Equation.
From exoyzonai.blob.core.windows.net
Titration Curve Labeled Buffer Region at Craig Johnson blog Titration Buffer Equation When a weak acid is titrated against a strong base, the ph of. in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or. Titration Buffer Equation.
From giorkvuqt.blob.core.windows.net
Vinegar Titration Curve at Gary Clark blog Titration Buffer Equation In chemistry, a buffer zone is a region where the ph of a solution remains constant. When a weak acid is titrated against a strong base, the ph of. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). in a buffer. Titration Buffer Equation.
From giofzfwwv.blob.core.windows.net
Acid Base Titration To Determine The Concentration at Latonya John blog Titration Buffer Equation When a weak acid is titrated against a strong base, the ph of. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In chemistry, a buffer zone is a region where the ph of a solution remains constant. ph = pk a + log [base / acid]. Titration Buffer Equation.
From exyuokede.blob.core.windows.net
Titration Graph Buffer at Mark Paras blog Titration Buffer Equation in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. for the titration of a weak base with a strong. Titration Buffer Equation.
From users.highland.edu
Buffers Titration Buffer Equation When a weak acid is titrated against a strong base, the ph of. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph <. Titration Buffer Equation.
From www.elettronicaveneta.com
Buffers and titration curves Elettronica S.p.A. Titration Buffer Equation ph = pk a + log [base / acid] is often the way you see it written on the internet. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Many q&a forums still lack the ability to make a. In chemistry, a buffer zone is a region. Titration Buffer Equation.
From saylordotorg.github.io
Aqueous AcidBase Equilibriums Titration Buffer Equation in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). When a weak acid is titrated against a strong base, the ph of. ph = pk a + log [base / acid] is often the way you see it written on the internet. Many q&a forums still lack. Titration Buffer Equation.
From www.chegg.com
Solved EXP. 5 BUFFERS, TITRATION CURVES, AND INDICATORS LAB Titration Buffer Equation for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). in this section, we will see how to perform calculations to. Titration Buffer Equation.
From exyybombn.blob.core.windows.net
Titration Curve Mathematical Formula at Josephine Christensen blog Titration Buffer Equation for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Many q&a forums still lack. Titration Buffer Equation.
From www.youtube.com
Buffer capacity Buffers, titrations, and solubility equilibria Titration Buffer Equation in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). When a weak acid is titrated against a strong base, the ph of. ph = pk a + log [base / acid] is often the way you see it written on the internet. the above equation works. Titration Buffer Equation.
From www.youtube.com
Titration and Buffers a test review YouTube Titration Buffer Equation in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In chemistry, a buffer zone is a region where the ph of a solution remains constant. When a. Titration Buffer Equation.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Titration Buffer Equation ph = pk a + log [base / acid] is often the way you see it written on the internet. in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). for the titration of a weak base with a strong acid, the ph curve is initially basic. Titration Buffer Equation.
From courses.lumenlearning.com
AcidBase Titrations General Chemistry Titration Buffer Equation in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). Many q&a forums still lack the ability to make a. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). In chemistry,. Titration Buffer Equation.
From www.youtube.com
GCII Buffers and Titrations practice problems. YouTube Titration Buffer Equation When a weak acid is titrated against a strong base, the ph of. Many q&a forums still lack the ability to make a. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. ph = pk a + log [base / acid] is often the way you see. Titration Buffer Equation.
From revise.im
Buffers and Neutralisation Revise.im Titration Buffer Equation in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). Many q&a forums still lack the ability to make a. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). the. Titration Buffer Equation.
From exycjgytb.blob.core.windows.net
What Is Titration In Chemistry Gcse at Michael Ignacio blog Titration Buffer Equation for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). When a weak acid is titrated against a strong base, the ph. Titration Buffer Equation.
From mavink.com
Buffer Region Titration Curve Titration Buffer Equation In chemistry, a buffer zone is a region where the ph of a solution remains constant. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). Many q&a forums still lack the ability to make a. in this section, we will see. Titration Buffer Equation.
From biotechnologymcq.com
Weak Acid Base PH Henderson Hasselbalch Equation Biological Buffer Titration Buffer Equation Many q&a forums still lack the ability to make a. When a weak acid is titrated against a strong base, the ph of. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. for the titration of a weak base with. Titration Buffer Equation.
From www.chegg.com
Solved Buffers, Titrations, pH Curves in Water Equations Titration Buffer Equation the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. ph = pk a + log [base / acid] is often the way you see it written on the internet. When a weak acid is titrated against a strong base, the ph of. in a buffer solution,. Titration Buffer Equation.
From webmis.highland.cc.il.us
AcidBase Titrations Titration Buffer Equation in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. When a weak acid is titrated against a strong base, the ph of. in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base. Titration Buffer Equation.
From www.studocu.com
4 CTEC233 Titrations buffers CTEC 233 CHEMICAL ANALYSIS Titration Buffer Equation In chemistry, a buffer zone is a region where the ph of a solution remains constant. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. When a weak acid is titrated against a strong base, the ph of. ph =. Titration Buffer Equation.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Titration Buffer Equation in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). ph = pk a + log [base / acid] is often the way you see it written on the internet. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid. Titration Buffer Equation.
From www.studocu.com
Chapter 14.614.7, 15 reading guide Ch 1414 Buffers & titrations Titration Buffer Equation in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). When a weak acid is titrated against a strong base, the ph of. In chemistry, a buffer zone is a region where the ph of a solution remains constant. ph = pk a + log [base / acid]. Titration Buffer Equation.
From hxebbzjce.blob.core.windows.net
Complexometric Titration Buffer Solution Role at Bruce Breece blog Titration Buffer Equation Many q&a forums still lack the ability to make a. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. When a weak acid is titrated against a strong base, the ph of. In chemistry, a buffer zone is a region where the ph of a solution remains constant.. Titration Buffer Equation.
From chemistryguru.com.sg
Titration Curve of Amino Acid Titration Buffer Equation Many q&a forums still lack the ability to make a. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. . Titration Buffer Equation.
From www.tessshebaylo.com
Chemical Equation For Sodium Carbonate And Hydrochloric Acid Tessshebaylo Titration Buffer Equation the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in a buffer solution, the weak acid (or the weak base) is in equilibrium with its conjugate base (or acid). in this section, we will see how to perform calculations to predict the ph at any point. Titration Buffer Equation.
From derangedphysiology.com
Buffers and buffering power Deranged Physiology Titration Buffer Equation ph = pk a + log [base / acid] is often the way you see it written on the internet. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. for the titration of a weak base with a strong acid, the ph curve is initially basic. Titration Buffer Equation.