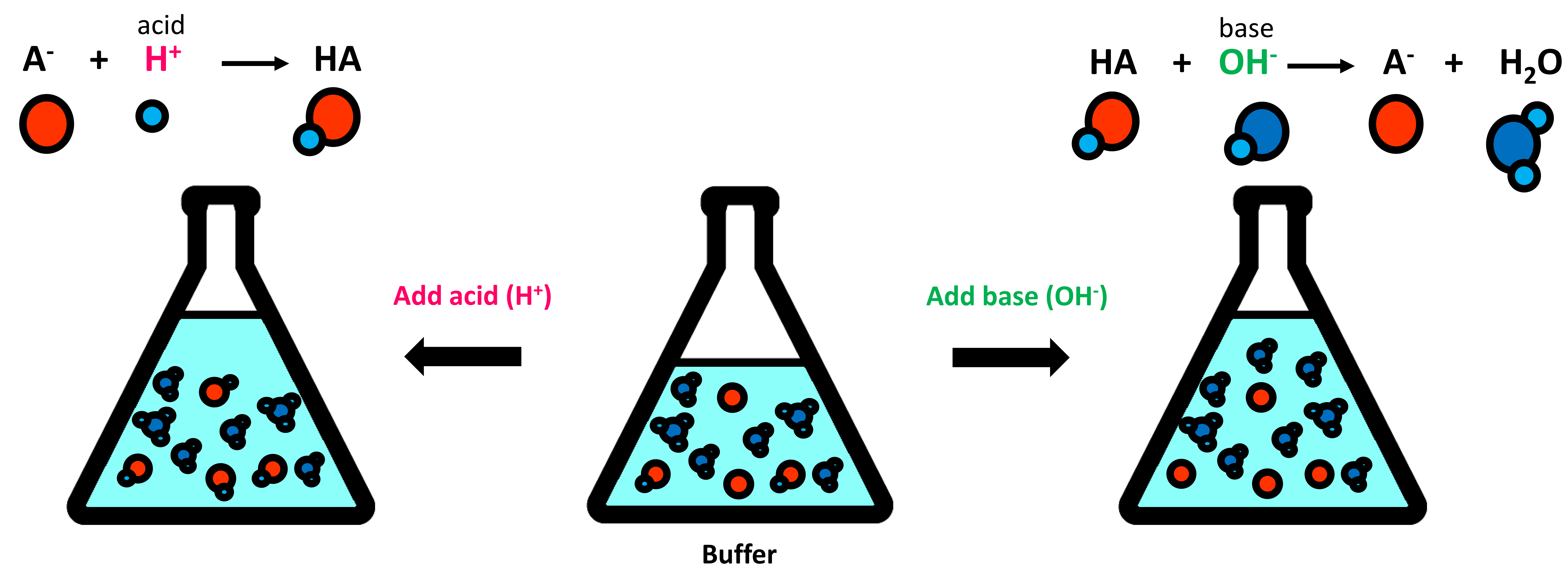
Why Does Buffer Capacity Increase With Concentration . The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity will be. The more concentrated the buffer mixture, the higher the buffer. By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. Now, buffer capacity can be defined as the. A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. The chemical composition of a buffer solution usually entails a weak acid or a weak base accompanied by its conjugate salt. For example, a buffer with [weak. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. Buffer capacity depends on the concentrations of the species in the solution;
from design.udlvirtual.edu.pe
A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. Now, buffer capacity can be defined as the. Buffer capacity depends on the concentrations of the species in the solution; The chemical composition of a buffer solution usually entails a weak acid or a weak base accompanied by its conjugate salt. By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity will be. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. For example, a buffer with [weak. The more concentrated the buffer mixture, the higher the buffer.
How Does A Circular Buffer Work Design Talk
Why Does Buffer Capacity Increase With Concentration The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity will be. By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. The chemical composition of a buffer solution usually entails a weak acid or a weak base accompanied by its conjugate salt. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. Now, buffer capacity can be defined as the. For example, a buffer with [weak. The more concentrated the buffer mixture, the higher the buffer. Buffer capacity depends on the concentrations of the species in the solution;
From twitchviral.com
Why Does Twitch Keep Buffering On My Phone? Why Does Buffer Capacity Increase With Concentration The chemical composition of a buffer solution usually entails a weak acid or a weak base accompanied by its conjugate salt. A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution. Why Does Buffer Capacity Increase With Concentration.
From www.numerade.com
SOLVEDWhy does the buffer capacity reach a maximum when pH=p Ka Why Does Buffer Capacity Increase With Concentration The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity will be. A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. The chemical composition of a buffer solution usually entails a weak acid or a. Why Does Buffer Capacity Increase With Concentration.
From design.udlvirtual.edu.pe
How Does A Circular Buffer Work Design Talk Why Does Buffer Capacity Increase With Concentration The more concentrated the buffer mixture, the higher the buffer. Now, buffer capacity can be defined as the. The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity will be. For example, a buffer with [weak. The buffer capacity is the amount of. Why Does Buffer Capacity Increase With Concentration.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Why Does Buffer Capacity Increase With Concentration The chemical composition of a buffer solution usually entails a weak acid or a weak base accompanied by its conjugate salt. A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. Now, buffer capacity can be defined as the. The more concentrated the buffer mixture, the higher the buffer. The buffer capacity is. Why Does Buffer Capacity Increase With Concentration.
From webmis.highland.cc.il.us
Buffers Why Does Buffer Capacity Increase With Concentration The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity will be. By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. The more concentrated the. Why Does Buffer Capacity Increase With Concentration.
From www.numerade.com
VIDEO solution Purely from a chemistry point of view, why does buffer Why Does Buffer Capacity Increase With Concentration Buffer capacity depends on the concentrations of the species in the solution; The chemical composition of a buffer solution usually entails a weak acid or a weak base accompanied by its conjugate salt. The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity. Why Does Buffer Capacity Increase With Concentration.
From www.lifewire.com
How to Avoid Buffering Issues When Streaming Video Why Does Buffer Capacity Increase With Concentration Now, buffer capacity can be defined as the. The more concentrated the buffer mixture, the higher the buffer. By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. The chemical composition of a buffer solution usually entails a weak acid or a weak. Why Does Buffer Capacity Increase With Concentration.
From thetechgorilla.com
Why Does Netflix Keep Buffering (Fixed!) The Tech Gorilla Why Does Buffer Capacity Increase With Concentration Now, buffer capacity can be defined as the. For example, a buffer with [weak. By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate. Why Does Buffer Capacity Increase With Concentration.
From www.chegg.com
Weak Acid solution used to prepare buffers a and b Why Does Buffer Capacity Increase With Concentration By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity will be. The buffer capacity is. Why Does Buffer Capacity Increase With Concentration.
From www.slideserve.com
PPT Chapter 13 Applications of Aqueous Equilibria PowerPoint Why Does Buffer Capacity Increase With Concentration The more concentrated the buffer mixture, the higher the buffer. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. The chemical composition of a buffer solution usually entails a weak acid or a weak base accompanied by its conjugate salt. By definition,. Why Does Buffer Capacity Increase With Concentration.
From solvedlib.com
What is the [Na2CO3] needed to mix with 0.2500 M H2CO… SolvedLib Why Does Buffer Capacity Increase With Concentration For example, a buffer with [weak. Now, buffer capacity can be defined as the. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. The more concentrated the buffer mixture, the higher the buffer. The higher the concentration of the weak acid and. Why Does Buffer Capacity Increase With Concentration.
From study.com
Identifying Buffer Capacity Chemistry Why Does Buffer Capacity Increase With Concentration Now, buffer capacity can be defined as the. By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. The buffer capacity is the amount of acid. Why Does Buffer Capacity Increase With Concentration.
From gamesmartz.com
Buffer Capacity Definition & Image GameSmartz Why Does Buffer Capacity Increase With Concentration The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity will be. By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. The more concentrated the. Why Does Buffer Capacity Increase With Concentration.
From www.youtube.com
Buffer capacity; making a buffer with a given pH value YouTube Why Does Buffer Capacity Increase With Concentration A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. The more concentrated the buffer mixture, the higher the buffer. Now, buffer capacity can be defined. Why Does Buffer Capacity Increase With Concentration.
From www.mysportscience.com
Sodium bicarbonate, cheap and effective? Why Does Buffer Capacity Increase With Concentration The chemical composition of a buffer solution usually entails a weak acid or a weak base accompanied by its conjugate salt. Buffer capacity depends on the concentrations of the species in the solution; The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity. Why Does Buffer Capacity Increase With Concentration.
From www.youtube.com
pH of buffer solutions the HendersonHasselbalch equation. YouTube Why Does Buffer Capacity Increase With Concentration By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. For example, a buffer with [weak. The more concentrated the buffer mixture, the higher the buffer. Buffer capacity depends on the concentrations of the species in the solution; The buffer capacity is the. Why Does Buffer Capacity Increase With Concentration.
From www.pinterest.com
Pin on Download Why Does Buffer Capacity Increase With Concentration The more concentrated the buffer mixture, the higher the buffer. The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity will be. By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be. Why Does Buffer Capacity Increase With Concentration.
From www.mheducation.com
What is McGraw Hill Virtual Labs? McGraw Hill Higher Education Why Does Buffer Capacity Increase With Concentration By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. Buffer capacity depends on the concentrations of the species in the solution; Now, buffer capacity can be defined as the. The buffer capacity is the amount of acid or base that can be. Why Does Buffer Capacity Increase With Concentration.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Why Does Buffer Capacity Increase With Concentration The more concentrated the buffer mixture, the higher the buffer. The chemical composition of a buffer solution usually entails a weak acid or a weak base accompanied by its conjugate salt. A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. For example, a buffer with [weak. The higher the concentration of the. Why Does Buffer Capacity Increase With Concentration.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free Why Does Buffer Capacity Increase With Concentration Now, buffer capacity can be defined as the. For example, a buffer with [weak. By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. The more concentrated the buffer mixture, the higher the buffer. A solution with more weak acid, [ha], has a. Why Does Buffer Capacity Increase With Concentration.
From exovxqbyx.blob.core.windows.net
Why Does My Prime Video Keep Buffering at Loretta Himes blog Why Does Buffer Capacity Increase With Concentration The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity will be. A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. Now, buffer capacity can be defined as the. By definition, buffer capacity is a. Why Does Buffer Capacity Increase With Concentration.
From www.numerade.com
VIDEO solution why does the buffering capacity of CSF is lower than Why Does Buffer Capacity Increase With Concentration Buffer capacity depends on the concentrations of the species in the solution; The more concentrated the buffer mixture, the higher the buffer. The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity will be. A solution with more weak acid, [ha], has a. Why Does Buffer Capacity Increase With Concentration.
From ar.inspiredpencil.com
Buffer Capacity Why Does Buffer Capacity Increase With Concentration By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. Buffer capacity depends on the concentrations of the species in the solution; The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer. Why Does Buffer Capacity Increase With Concentration.
From www.skybest.com
What is buffering on TV? How to stop buffering when streaming on TV Why Does Buffer Capacity Increase With Concentration The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity will be. Now, buffer capacity can be defined as the. A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. For example, a buffer with [weak.. Why Does Buffer Capacity Increase With Concentration.
From theaterdiy.com
Why Does My Spectrum Keep Buffering? Find Solutions Now! (2024) Why Does Buffer Capacity Increase With Concentration By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. Now, buffer capacity can be defined. Why Does Buffer Capacity Increase With Concentration.
From facts.net
13 Enigmatic Facts About Buffer Capacity Why Does Buffer Capacity Increase With Concentration Buffer capacity depends on the concentrations of the species in the solution; A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. The more concentrated the. Why Does Buffer Capacity Increase With Concentration.
From theaterdiy.com
Why Does Spectrum TV Keep Buffering? (2024) Why Does Buffer Capacity Increase With Concentration A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. The chemical composition of a buffer solution usually entails a weak acid or a weak base accompanied by its conjugate salt. For example, a buffer with [weak. By definition, buffer capacity is a measure of the number of moles of strong acid or. Why Does Buffer Capacity Increase With Concentration.
From www.chegg.com
Solved 1. How does the concentration of the buffer affect Why Does Buffer Capacity Increase With Concentration The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. Now, buffer capacity can be defined as the. The more concentrated the buffer mixture, the higher the buffer. For example, a buffer with [weak. The chemical composition of a buffer solution usually entails. Why Does Buffer Capacity Increase With Concentration.
From ppt-online.org
Solutions. Acidbase equilibrium in biological systems презентация онлайн Why Does Buffer Capacity Increase With Concentration The chemical composition of a buffer solution usually entails a weak acid or a weak base accompanied by its conjugate salt. By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. For example, a buffer with [weak. The more concentrated the buffer mixture,. Why Does Buffer Capacity Increase With Concentration.
From thetechgorilla.com
Why does Netflix keep buffering (This works!) The Tech Gorilla Why Does Buffer Capacity Increase With Concentration The chemical composition of a buffer solution usually entails a weak acid or a weak base accompanied by its conjugate salt. For example, a buffer with [weak. By definition, buffer capacity is a measure of the number of moles of strong acid or strong base that can be added to a buffer system without. Buffer capacity depends on the concentrations. Why Does Buffer Capacity Increase With Concentration.
From ar.inspiredpencil.com
Buffer Capacity Why Does Buffer Capacity Increase With Concentration The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. Now, buffer capacity can be defined as the. By definition, buffer capacity is a measure of. Why Does Buffer Capacity Increase With Concentration.
From robots.net
Why Does My Tablet Keep Buffering Why Does Buffer Capacity Increase With Concentration The more concentrated the buffer mixture, the higher the buffer. The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity will be. Buffer capacity depends on the concentrations of the species in the solution; The chemical composition of a buffer solution usually entails. Why Does Buffer Capacity Increase With Concentration.
From advicefortech.com
Why Does My Television Keep Buffering? Why Does Buffer Capacity Increase With Concentration Buffer capacity depends on the concentrations of the species in the solution; A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. Now, buffer capacity can. Why Does Buffer Capacity Increase With Concentration.
From ar.inspiredpencil.com
Riparian Buffer Why Does Buffer Capacity Increase With Concentration The more concentrated the buffer mixture, the higher the buffer. A solution with more weak acid, [ha], has a higher buffer capacity for addition of strong base. Now, buffer capacity can be defined as the. The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher. Why Does Buffer Capacity Increase With Concentration.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online Why Does Buffer Capacity Increase With Concentration The more concentrated the buffer mixture, the higher the buffer. Now, buffer capacity can be defined as the. The higher the concentration of the weak acid and the conjugate base (or a weak base and a conjugate acid) a buffer has, the higher its capacity will be. For example, a buffer with [weak. A solution with more weak acid, [ha],. Why Does Buffer Capacity Increase With Concentration.