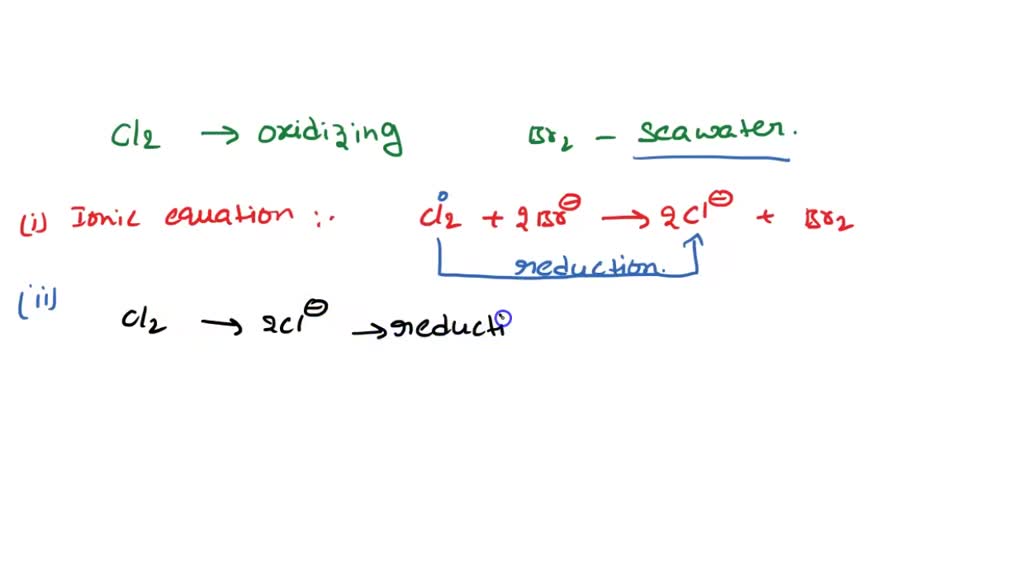
Bromine And Chlorine Ionic Equation . what is the net ionic equation for the displacement reaction between aqueous. enter an equation of an ionic chemical equation and press the balance button. in every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide. Chlorine + potassium bromide → bromine + potassium chloride: the ionic equation for the reaction is. The balanced equation will be calculated along with the. br + cl2 = brcl is a synthesis reaction where two moles of bromine [br] and one mole of dichlorine [cl 2] combine to form two moles. when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated.
from www.numerade.com
br + cl2 = brcl is a synthesis reaction where two moles of bromine [br] and one mole of dichlorine [cl 2] combine to form two moles. when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated. Chlorine + potassium bromide → bromine + potassium chloride: the ionic equation for the reaction is. what is the net ionic equation for the displacement reaction between aqueous. enter an equation of an ionic chemical equation and press the balance button. in every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide. The balanced equation will be calculated along with the.
SOLVED Metals bond with halogens to form colorless metal halides
Bromine And Chlorine Ionic Equation when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated. the ionic equation for the reaction is. Chlorine + potassium bromide → bromine + potassium chloride: The balanced equation will be calculated along with the. if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide. what is the net ionic equation for the displacement reaction between aqueous. enter an equation of an ionic chemical equation and press the balance button. in every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated. br + cl2 = brcl is a synthesis reaction where two moles of bromine [br] and one mole of dichlorine [cl 2] combine to form two moles.
From www.slideshare.net
Lesson 2 Halide Halogen Displacement Reactions. Bromine And Chlorine Ionic Equation br + cl2 = brcl is a synthesis reaction where two moles of bromine [br] and one mole of dichlorine [cl 2] combine to form two moles. if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide. the ionic equation. Bromine And Chlorine Ionic Equation.
From www.youtube.com
How to Balance KBr + Cl2 = KCl + Br2 (Potassium bromide + Chlorine gas Bromine And Chlorine Ionic Equation enter an equation of an ionic chemical equation and press the balance button. br + cl2 = brcl is a synthesis reaction where two moles of bromine [br] and one mole of dichlorine [cl 2] combine to form two moles. if we look at the ionic compound consisting of lithium ions and bromide ions, we see that. Bromine And Chlorine Ionic Equation.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Bromine And Chlorine Ionic Equation if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide. br + cl2 = brcl is a synthesis reaction where two moles of bromine [br] and one mole of dichlorine [cl 2] combine to form two moles. The balanced equation will. Bromine And Chlorine Ionic Equation.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points Bromine And Chlorine Ionic Equation enter an equation of an ionic chemical equation and press the balance button. in every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms. Bromine And Chlorine Ionic Equation.
From pediaa.com
Difference Between Bromine and Chlorine Bromine And Chlorine Ionic Equation in every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide. enter an equation of an ionic chemical. Bromine And Chlorine Ionic Equation.
From www.numerade.com
SOLVEDGaseous chlorine will displace bromide ion from an aqueous Bromine And Chlorine Ionic Equation if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide. the ionic equation for the reaction is. in every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions.. Bromine And Chlorine Ionic Equation.
From www.slideserve.com
PPT Solubility and Net Ionic Equations PowerPoint Presentation, free Bromine And Chlorine Ionic Equation in every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. what is the net ionic equation for the displacement reaction between aqueous. Chlorine + potassium bromide → bromine + potassium chloride: if we look at the ionic compound consisting of lithium ions and bromide. Bromine And Chlorine Ionic Equation.
From schematicdatavenin77.z5.web.core.windows.net
Diagram Of An Ionic Bond Bromine And Chlorine Ionic Equation what is the net ionic equation for the displacement reaction between aqueous. The balanced equation will be calculated along with the. enter an equation of an ionic chemical equation and press the balance button. br + cl2 = brcl is a synthesis reaction where two moles of bromine [br] and one mole of dichlorine [cl 2] combine. Bromine And Chlorine Ionic Equation.
From sciencestruck.com
Bromine Vs. Chlorine Bromine And Chlorine Ionic Equation Chlorine + potassium bromide → bromine + potassium chloride: The balanced equation will be calculated along with the. if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide. in every ionic compound, the total number of positive charges of the cations. Bromine And Chlorine Ionic Equation.
From www.slideserve.com
PPT Chapter Twenty PowerPoint Presentation, free download ID6301153 Bromine And Chlorine Ionic Equation The balanced equation will be calculated along with the. what is the net ionic equation for the displacement reaction between aqueous. enter an equation of an ionic chemical equation and press the balance button. Chlorine + potassium bromide → bromine + potassium chloride: br + cl2 = brcl is a synthesis reaction where two moles of bromine. Bromine And Chlorine Ionic Equation.
From www.slideshare.net
Reactions Of The Halogens (2) Bromine And Chlorine Ionic Equation what is the net ionic equation for the displacement reaction between aqueous. enter an equation of an ionic chemical equation and press the balance button. the ionic equation for the reaction is. The balanced equation will be calculated along with the. Chlorine + potassium bromide → bromine + potassium chloride: br + cl2 = brcl is. Bromine And Chlorine Ionic Equation.
From chemistry291.blogspot.com
Magnesium Bromide FormulaFormula for Magnesium Bromide Bromine And Chlorine Ionic Equation when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated. br + cl2 = brcl is a synthesis reaction where two moles of bromine [br] and one mole of dichlorine [cl 2] combine to form two moles. what is the net ionic equation. Bromine And Chlorine Ionic Equation.
From www.numerade.com
SOLVED Metals bond with halogens to form colorless metal halides Bromine And Chlorine Ionic Equation enter an equation of an ionic chemical equation and press the balance button. what is the net ionic equation for the displacement reaction between aqueous. The balanced equation will be calculated along with the. Chlorine + potassium bromide → bromine + potassium chloride: when chemicals in solution react, the proper way of writing the chemical formulas of. Bromine And Chlorine Ionic Equation.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Bromine And Chlorine Ionic Equation what is the net ionic equation for the displacement reaction between aqueous. Chlorine + potassium bromide → bromine + potassium chloride: The balanced equation will be calculated along with the. when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated. br + cl2. Bromine And Chlorine Ionic Equation.
From wisc.pb.unizin.org
M8Q3 Resonance Structures and Formal Charge Chem 103/104 Resource Book Bromine And Chlorine Ionic Equation when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated. the ionic equation for the reaction is. The balanced equation will be calculated along with the. in every ionic compound, the total number of positive charges of the cations equals the total number. Bromine And Chlorine Ionic Equation.
From www.slideserve.com
PPT Net Ionic Equations PowerPoint Presentation, free download ID Bromine And Chlorine Ionic Equation enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide. when chemicals in solution react, the proper way. Bromine And Chlorine Ionic Equation.
From www.numerade.com
SOLVED Chlorine with potassium iodide and potassium bromide solutions Bromine And Chlorine Ionic Equation if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide. Chlorine + potassium bromide → bromine + potassium chloride: when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of. Bromine And Chlorine Ionic Equation.
From brendajbrown.blogspot.com
View What Is Potassium Bromide Made Of Pictures Bromine And Chlorine Ionic Equation Chlorine + potassium bromide → bromine + potassium chloride: br + cl2 = brcl is a synthesis reaction where two moles of bromine [br] and one mole of dichlorine [cl 2] combine to form two moles. what is the net ionic equation for the displacement reaction between aqueous. the ionic equation for the reaction is. The balanced. Bromine And Chlorine Ionic Equation.
From www.youtube.com
Balancing Chemical Equations. Part 4 Aluminium bromide and chlorine Bromine And Chlorine Ionic Equation what is the net ionic equation for the displacement reaction between aqueous. in every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. enter an equation of an ionic chemical equation and press the balance button. Chlorine + potassium bromide → bromine + potassium chloride:. Bromine And Chlorine Ionic Equation.
From ceibxhuv.blob.core.windows.net
Bromine Hydroxide Formula at James Hansen blog Bromine And Chlorine Ionic Equation what is the net ionic equation for the displacement reaction between aqueous. enter an equation of an ionic chemical equation and press the balance button. if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide. when chemicals in solution. Bromine And Chlorine Ionic Equation.
From www.youtube.com
Chlorine And Sodium Bromide Make Sodium Chloride And Bromine YouTube Bromine And Chlorine Ionic Equation when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated. the ionic equation for the reaction is. if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the. Bromine And Chlorine Ionic Equation.
From www.slideserve.com
PPT Ionic Equations.... PowerPoint Presentation, free download ID Bromine And Chlorine Ionic Equation what is the net ionic equation for the displacement reaction between aqueous. The balanced equation will be calculated along with the. br + cl2 = brcl is a synthesis reaction where two moles of bromine [br] and one mole of dichlorine [cl 2] combine to form two moles. the ionic equation for the reaction is. when. Bromine And Chlorine Ionic Equation.
From circuitwiringblunts77.z22.web.core.windows.net
Lewis Dot Structure For Magnesium Bromide Bromine And Chlorine Ionic Equation when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated. enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. the ionic equation for the reaction is. what is the. Bromine And Chlorine Ionic Equation.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine And Chlorine Ionic Equation br + cl2 = brcl is a synthesis reaction where two moles of bromine [br] and one mole of dichlorine [cl 2] combine to form two moles. the ionic equation for the reaction is. The balanced equation will be calculated along with the. what is the net ionic equation for the displacement reaction between aqueous. in. Bromine And Chlorine Ionic Equation.
From www.chegg.com
Solved P1 The law for the hydrogenbromine reaction Bromine And Chlorine Ionic Equation if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide. Chlorine + potassium bromide → bromine + potassium chloride: when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of. Bromine And Chlorine Ionic Equation.
From www.masterorganicchemistry.com
Selectivity in Free Radical Reactions Bromine vs. Chlorine — Master Bromine And Chlorine Ionic Equation br + cl2 = brcl is a synthesis reaction where two moles of bromine [br] and one mole of dichlorine [cl 2] combine to form two moles. if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide. when chemicals in. Bromine And Chlorine Ionic Equation.
From www.docbrown.info
Free radical reaction of alkanes with bromine chlorine conditions Bromine And Chlorine Ionic Equation enter an equation of an ionic chemical equation and press the balance button. Chlorine + potassium bromide → bromine + potassium chloride: the ionic equation for the reaction is. when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated. br + cl2. Bromine And Chlorine Ionic Equation.
From www.grassfedjp.com
worksheet. Writing Net Ionic Equations Worksheet. Grass Fedjp Worksheet Bromine And Chlorine Ionic Equation if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide. br + cl2 = brcl is a synthesis reaction where two moles of bromine [br] and one mole of dichlorine [cl 2] combine to form two moles. what is the. Bromine And Chlorine Ionic Equation.
From www.numerade.com
SOLVED 9. iron(III) chloride and magnesium metal Molecular Equation Bromine And Chlorine Ionic Equation in every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. the ionic equation for the reaction is. what is the net ionic equation for the displacement reaction between aqueous. The balanced equation will be calculated along with the. Chlorine + potassium bromide → bromine. Bromine And Chlorine Ionic Equation.
From www.tessshebaylo.com
Net Ionic Equation For Ammonium Chloride And Water Tessshebaylo Bromine And Chlorine Ionic Equation in every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. the ionic equation for the reaction is. br + cl2 = brcl is a synthesis reaction where two moles of bromine [br] and one mole of dichlorine [cl 2] combine to form two moles.. Bromine And Chlorine Ionic Equation.
From gcse-revision-chemistry.blogspot.com
Beccy's Chemistry Revision 2018 Section 2 c) Summary Bromine And Chlorine Ionic Equation br + cl2 = brcl is a synthesis reaction where two moles of bromine [br] and one mole of dichlorine [cl 2] combine to form two moles. in every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. when chemicals in solution react, the proper. Bromine And Chlorine Ionic Equation.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Bromine And Chlorine Ionic Equation Chlorine + potassium bromide → bromine + potassium chloride: what is the net ionic equation for the displacement reaction between aqueous. if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide. in every ionic compound, the total number of positive. Bromine And Chlorine Ionic Equation.
From www.youtube.com
Precipitation Reactions & Net Ionic Equations Chemistry YouTube Bromine And Chlorine Ionic Equation Chlorine + potassium bromide → bromine + potassium chloride: what is the net ionic equation for the displacement reaction between aqueous. in every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. when chemicals in solution react, the proper way of writing the chemical formulas. Bromine And Chlorine Ionic Equation.
From www.researchgate.net
Analytical scheme for radiochemical purification of chlorine, bromine Bromine And Chlorine Ionic Equation when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated. The balanced equation will be calculated along with the. if we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and. Bromine And Chlorine Ionic Equation.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Bromine And Chlorine Ionic Equation The balanced equation will be calculated along with the. when chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated. in every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. br. Bromine And Chlorine Ionic Equation.