Boiling Point Of Distilled Water With Salt . If you add salt to water, you raise the boiling point, or the temperature at which water boils. Adding salt does not lower the boiling point of water. It's true that part of why salt dissolves well in. My understanding is that the boiling point will be higher, thus lengthening the process (obtaining boiling water), but at the same time, the. The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of water. Adding salt to water results in a phenomenon called boiling point elevation. Actually, the opposite is true. For example, in denver, colorado, the boiling point is about 95. Similarly, you also lower its freezing point. The boiling point of water increases when salt is dissolved in it. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. Updated on july 13, 2024. At at high altitudes the lower pressure makes the boiling point several degrees lower. This phenomenon is known as boiling point. This gives salt water a higher.
from www.watersmartsystems.com
At at high altitudes the lower pressure makes the boiling point several degrees lower. It's true that part of why salt dissolves well in. If you add salt to water, you raise the boiling point, or the temperature at which water boils. This process is an example of boiling point elevation, and it's not exclusive to water. Adding salt does not lower the boiling point of water. The boiling point of water increases when salt is dissolved in it. Actually, the opposite is true. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. This phenomenon is known as boiling point. Any solute in water raises the boiling point, so long as the solute stays in the liquid water.
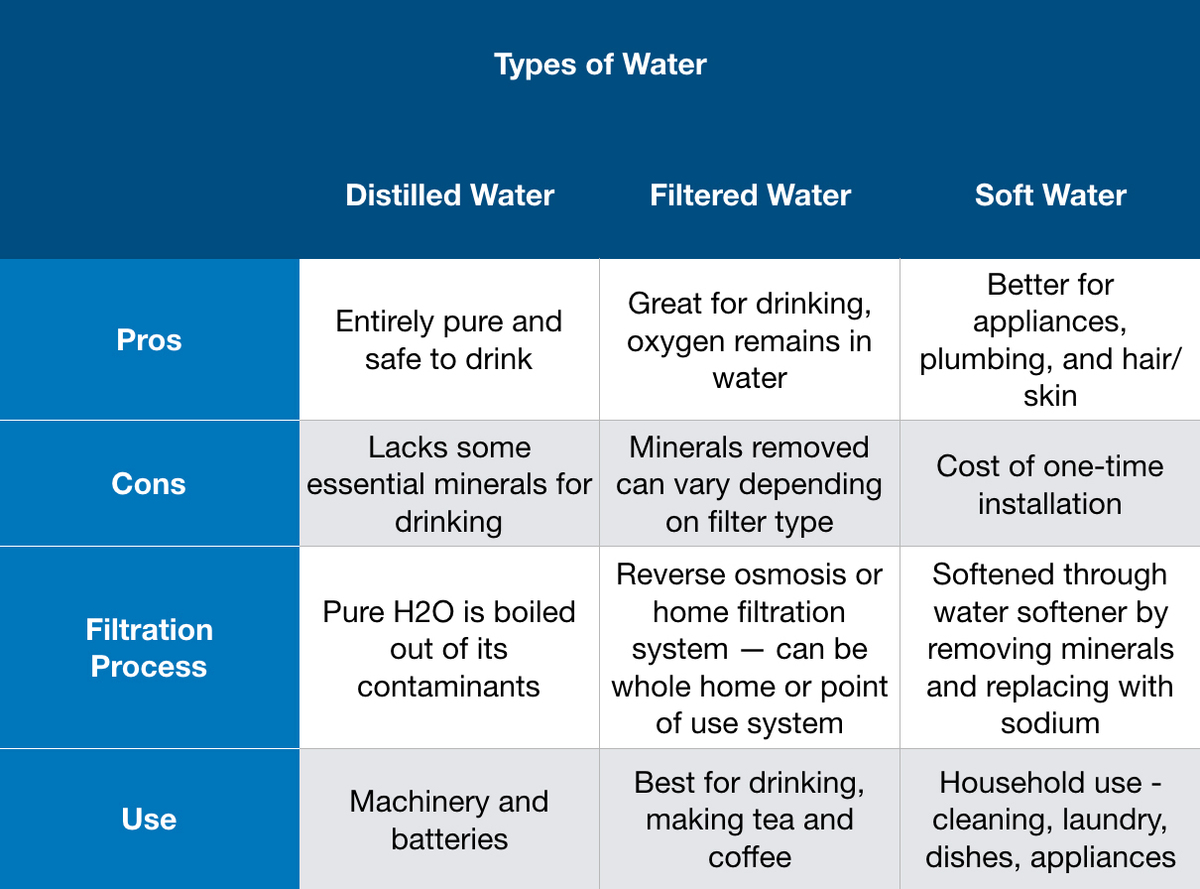
Distilled Water vs Water Softener vs Filtered Water Detailed
Boiling Point Of Distilled Water With Salt Similarly, you also lower its freezing point. If you add salt to water, you raise the boiling point, or the temperature at which water boils. This phenomenon is known as boiling point. This gives salt water a higher. My understanding is that the boiling point will be higher, thus lengthening the process (obtaining boiling water), but at the same time, the. Similarly, you also lower its freezing point. At at high altitudes the lower pressure makes the boiling point several degrees lower. The boiling point of water increases when salt is dissolved in it. This process is an example of boiling point elevation, and it's not exclusive to water. For example, in denver, colorado, the boiling point is about 95. The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of water. Actually, the opposite is true. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. Adding salt to water results in a phenomenon called boiling point elevation. Updated on july 13, 2024. Adding salt does not lower the boiling point of water.
From brainly.in
Describe an activity to determine the boiling point of water. Brainly.in Boiling Point Of Distilled Water With Salt For example, in denver, colorado, the boiling point is about 95. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. Actually, the opposite is true. This process is an example of boiling point elevation, and it's not exclusive to water.. Boiling Point Of Distilled Water With Salt.
From webapi.bu.edu
🌱 How does salt affect boiling point. To investigate the effect of the Boiling Point Of Distilled Water With Salt Adding salt does not lower the boiling point of water. This process is an example of boiling point elevation, and it's not exclusive to water. If you add salt to water, you raise the boiling point, or the temperature at which water boils. This phenomenon is known as boiling point. It's true that part of why salt dissolves well in.. Boiling Point Of Distilled Water With Salt.
From www.chegg.com
Solved 21°C Distilled Water Temperature Mass of Empty 100mL Boiling Point Of Distilled Water With Salt This process is an example of boiling point elevation, and it's not exclusive to water. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. If you add salt to water, you raise the boiling point, or the temperature at which water boils. At at high altitudes the lower pressure makes the. Boiling Point Of Distilled Water With Salt.
From www.perkins.org
Can the Boiling Point of Water be Changed? Perkins School for the Blind Boiling Point Of Distilled Water With Salt Similarly, you also lower its freezing point. It's true that part of why salt dissolves well in. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. This phenomenon is known as boiling point. This process is an example of boiling. Boiling Point Of Distilled Water With Salt.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Boiling Point Of Distilled Water With Salt When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. Actually, the opposite is true. Adding salt to water results in a phenomenon called boiling point elevation. Updated on july 13, 2024. The temperature needed to boil will increase by about. Boiling Point Of Distilled Water With Salt.
From www.chemicals.co.uk
What’s the Difference Between Distilled & Ultrapure Water? The Boiling Point Of Distilled Water With Salt At at high altitudes the lower pressure makes the boiling point several degrees lower. This process is an example of boiling point elevation, and it's not exclusive to water. Updated on july 13, 2024. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. The temperature needed to boil will increase by. Boiling Point Of Distilled Water With Salt.
From georgeperestheoxiia4.blogspot.com
Diy Distilled Water Machine 3 Ways To Make Distilled Water Wikihow Boiling Point Of Distilled Water With Salt This phenomenon is known as boiling point. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. Adding salt does not lower the boiling point of water. This gives salt water a higher. The temperature needed to boil will increase by. Boiling Point Of Distilled Water With Salt.
From www.watersmartsystems.com
Distilled Water vs Water Softener vs Filtered Water Detailed Boiling Point Of Distilled Water With Salt This process is an example of boiling point elevation, and it's not exclusive to water. This phenomenon is known as boiling point. My understanding is that the boiling point will be higher, thus lengthening the process (obtaining boiling water), but at the same time, the. Any solute in water raises the boiling point, so long as the solute stays in. Boiling Point Of Distilled Water With Salt.
From www.numerade.com
SOLVED A mixture of two miscible liquids with a widely different Boiling Point Of Distilled Water With Salt Any solute in water raises the boiling point, so long as the solute stays in the liquid water. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. Adding salt does not lower the boiling point of water. This gives salt. Boiling Point Of Distilled Water With Salt.
From www.cookist.com
Why Do Some People Add Salt To Boiling Water? Boiling Point Of Distilled Water With Salt For example, in denver, colorado, the boiling point is about 95. The boiling point of water increases when salt is dissolved in it. Adding salt to water results in a phenomenon called boiling point elevation. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. My understanding is that the boiling point. Boiling Point Of Distilled Water With Salt.
From askfilo.com
The normal boiling point of pure water is 373.15 K. Calculate the boiling.. Boiling Point Of Distilled Water With Salt When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. Updated on july 13, 2024. Similarly, you also lower its freezing point. Adding salt does not lower the boiling point of water. For example, in denver, colorado, the boiling point is. Boiling Point Of Distilled Water With Salt.
From lessonlangdonkirks.z21.web.core.windows.net
Adding Salt In Water Boiling Point Boiling Point Of Distilled Water With Salt Updated on july 13, 2024. At at high altitudes the lower pressure makes the boiling point several degrees lower. It's true that part of why salt dissolves well in. This process is an example of boiling point elevation, and it's not exclusive to water. Actually, the opposite is true. Similarly, you also lower its freezing point. For example, in denver,. Boiling Point Of Distilled Water With Salt.
From www.cookist.com
Why Do Some People Add Salt To Boiling Water? Boiling Point Of Distilled Water With Salt Actually, the opposite is true. Similarly, you also lower its freezing point. Updated on july 13, 2024. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. Adding salt to water results in a phenomenon called boiling point elevation. The temperature. Boiling Point Of Distilled Water With Salt.
From www.reference.com
What Is the Boiling Point of Distilled Water? Boiling Point Of Distilled Water With Salt Updated on july 13, 2024. This process is an example of boiling point elevation, and it's not exclusive to water. The boiling point of water increases when salt is dissolved in it. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings. Boiling Point Of Distilled Water With Salt.
From chem.libretexts.org
1.2 Classification of Matter Chemistry LibreTexts Boiling Point Of Distilled Water With Salt The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of water. This phenomenon is known as boiling point. Adding salt to water results in a phenomenon called boiling point elevation. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. Adding. Boiling Point Of Distilled Water With Salt.
From www.thoughtco.com
Why Adding Salt to Water Increases the Boiling Point Boiling Point Of Distilled Water With Salt If you add salt to water, you raise the boiling point, or the temperature at which water boils. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. Adding salt does not lower the boiling point of water. This process is. Boiling Point Of Distilled Water With Salt.
From www.scribd.com
Preparation of Distilled Water Distillation Boiling Boiling Point Of Distilled Water With Salt At at high altitudes the lower pressure makes the boiling point several degrees lower. The boiling point of water increases when salt is dissolved in it. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. Adding salt to water results in a phenomenon called boiling point elevation. For example, in denver,. Boiling Point Of Distilled Water With Salt.
From sciencenotes.org
Can You Drink Distilled Water? Is It Safe? Boiling Point Of Distilled Water With Salt Actually, the opposite is true. It's true that part of why salt dissolves well in. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. If you add salt to water, you raise the boiling point, or the temperature at which water boils. This gives salt water a higher. Adding salt to. Boiling Point Of Distilled Water With Salt.
From www.vecteezy.com
H2o mollecule Properties and Chemical Compound Structure water consist Boiling Point Of Distilled Water With Salt If you add salt to water, you raise the boiling point, or the temperature at which water boils. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. My understanding is that the boiling point will be higher, thus lengthening the process (obtaining boiling water), but at the same time, the. Updated. Boiling Point Of Distilled Water With Salt.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Boiling Point Of Distilled Water With Salt Adding salt does not lower the boiling point of water. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Updated on july 13, 2024. At at high altitudes the lower pressure makes the boiling point several degrees lower. Adding salt to water results in a phenomenon called boiling point elevation. For. Boiling Point Of Distilled Water With Salt.
From studylib.net
Boiling Point Fresh vs Salt Water Lab Boiling Point Of Distilled Water With Salt If you add salt to water, you raise the boiling point, or the temperature at which water boils. This process is an example of boiling point elevation, and it's not exclusive to water. This phenomenon is known as boiling point. The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram. Boiling Point Of Distilled Water With Salt.
From madalyn-has-dorsey.blogspot.com
Boiling Point of Water MadalynhasDorsey Boiling Point Of Distilled Water With Salt The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of water. Updated on july 13, 2024. At at high altitudes the lower pressure makes the boiling point several degrees lower. When salt is added, it makes it harder for the water molecules to escape from the pot and enter. Boiling Point Of Distilled Water With Salt.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Boiling Point Of Distilled Water With Salt This gives salt water a higher. At at high altitudes the lower pressure makes the boiling point several degrees lower. For example, in denver, colorado, the boiling point is about 95. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said.. Boiling Point Of Distilled Water With Salt.
From www.tessshebaylo.com
Chemical Equation For Water Boiling Tessshebaylo Boiling Point Of Distilled Water With Salt It's true that part of why salt dissolves well in. My understanding is that the boiling point will be higher, thus lengthening the process (obtaining boiling water), but at the same time, the. Adding salt does not lower the boiling point of water. When salt is added, it makes it harder for the water molecules to escape from the pot. Boiling Point Of Distilled Water With Salt.
From www.nagwa.com
Question Video Identifying in Which Part of the Apparatus Salt Would Boiling Point Of Distilled Water With Salt If you add salt to water, you raise the boiling point, or the temperature at which water boils. This gives salt water a higher. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. The temperature needed to boil will increase. Boiling Point Of Distilled Water With Salt.
From www.youtube.com
Does Salt Water Boil Faster? Experiment YouTube Boiling Point Of Distilled Water With Salt For example, in denver, colorado, the boiling point is about 95. It's true that part of why salt dissolves well in. The boiling point of water increases when salt is dissolved in it. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils,. Boiling Point Of Distilled Water With Salt.
From www.numerade.com
SOLVED A student observed that the boiling point of distilled water is Boiling Point Of Distilled Water With Salt At at high altitudes the lower pressure makes the boiling point several degrees lower. Similarly, you also lower its freezing point. Updated on july 13, 2024. Adding salt does not lower the boiling point of water. It's true that part of why salt dissolves well in. If you add salt to water, you raise the boiling point, or the temperature. Boiling Point Of Distilled Water With Salt.
From mconry.uneportfolio.org
The Art of Cooking With Pasta Michael Conry's Cooking and College Boiling Point Of Distilled Water With Salt Any solute in water raises the boiling point, so long as the solute stays in the liquid water. At at high altitudes the lower pressure makes the boiling point several degrees lower. The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of water. My understanding is that the boiling. Boiling Point Of Distilled Water With Salt.
From www.pinterest.com
Is Boiled Water and Distilled Water The Same? (Not Exactly, Here’s Why Boiling Point Of Distilled Water With Salt Any solute in water raises the boiling point, so long as the solute stays in the liquid water. For example, in denver, colorado, the boiling point is about 95. This phenomenon is known as boiling point. At at high altitudes the lower pressure makes the boiling point several degrees lower. Updated on july 13, 2024. If you add salt to. Boiling Point Of Distilled Water With Salt.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Boiling Point Of Distilled Water With Salt Any solute in water raises the boiling point, so long as the solute stays in the liquid water. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. This phenomenon is known as boiling point. For example, in denver, colorado, the. Boiling Point Of Distilled Water With Salt.
From lisassimplelife.com
Distilled waterBoiling Process for Distilled Water Lisa's Simple Life Boiling Point Of Distilled Water With Salt Actually, the opposite is true. It's true that part of why salt dissolves well in. The boiling point of water increases when salt is dissolved in it. Adding salt does not lower the boiling point of water. This phenomenon is known as boiling point. Similarly, you also lower its freezing point. Any solute in water raises the boiling point, so. Boiling Point Of Distilled Water With Salt.
From mavink.com
Melting And Boiling Point Chart Boiling Point Of Distilled Water With Salt It's true that part of why salt dissolves well in. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. For example, in denver, colorado, the boiling point is about 95. Updated on july 13, 2024. When salt is added, it makes it harder for the water molecules to escape from the. Boiling Point Of Distilled Water With Salt.
From www.bartleby.com
Answered Mass of empty 100 mL beaker Mass of… bartleby Boiling Point Of Distilled Water With Salt For example, in denver, colorado, the boiling point is about 95. It's true that part of why salt dissolves well in. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. This phenomenon is known as boiling point. This gives salt water a higher. Actually, the opposite is true. The temperature needed. Boiling Point Of Distilled Water With Salt.
From www.scribd.com
The Difference Between Distilled Water and Salt Water Solution in Terms Boiling Point Of Distilled Water With Salt Actually, the opposite is true. For example, in denver, colorado, the boiling point is about 95. The temperature needed to boil will increase by about 0.5 c for every 58 grams of dissolved salt per kilogram of water. This phenomenon is known as boiling point. The boiling point of water increases when salt is dissolved in it. Any solute in. Boiling Point Of Distilled Water With Salt.
From www.meritnation.com
Explain experiment on melting point ,boiling point Science Matter Boiling Point Of Distilled Water With Salt For example, in denver, colorado, the boiling point is about 95. This gives salt water a higher. Adding salt to water results in a phenomenon called boiling point elevation. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. This phenomenon is known as boiling point. Actually, the opposite is true. When. Boiling Point Of Distilled Water With Salt.