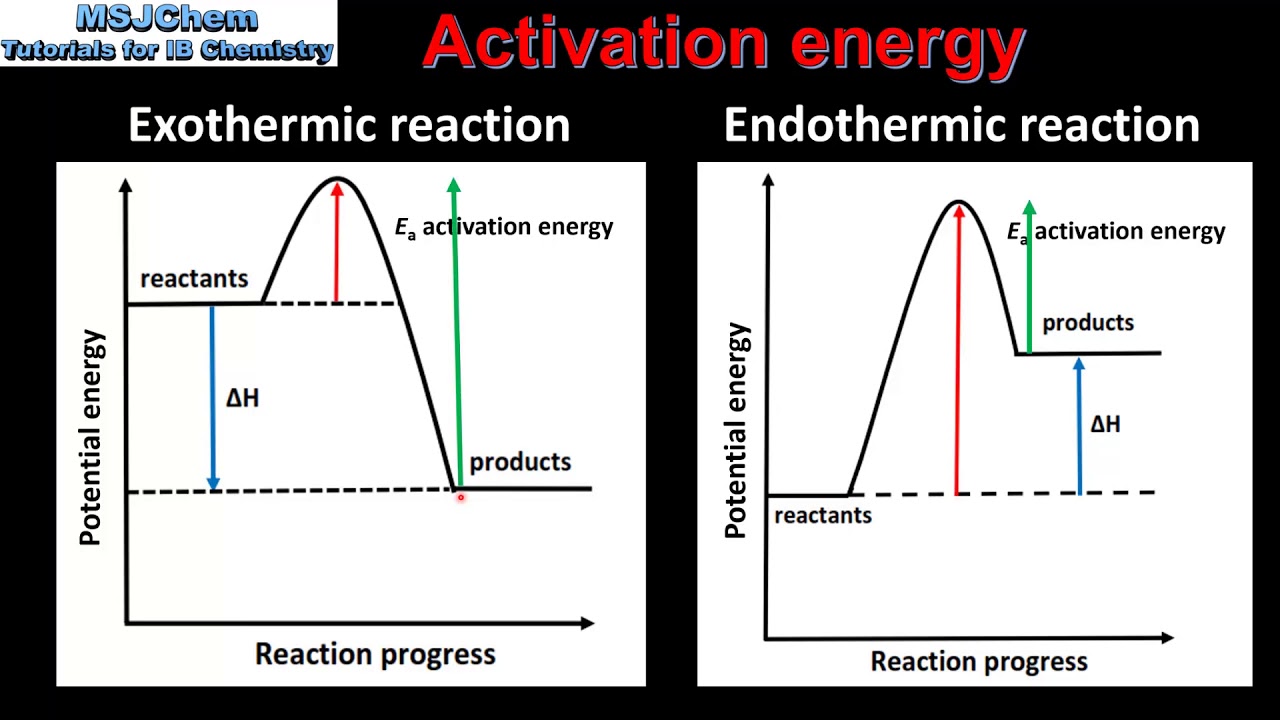
Endothermic Reaction Activation Energy Graph . The energy changes that occur in reactions involving a multistep mechanism can be shown in a graph. It is possible to represent the endothermic reaction in the form of a graph called the energy diagram. This is because energy is taken in from the. The energy level increases in an endothermic reaction. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. Lowering the activation energy of a reaction by a catalyst. The energy difference between reactants and the transition state is called the activation energy, δg ‡, and determines how rapidly the reaction. The potential energy of the reaction is plotted along the vertical axis,. This graph compares potential energy diagrams. This is asking you to draw a potential energy diagram for an endothermic reaction.
from www.youtube.com
The energy difference between reactants and the transition state is called the activation energy, δg ‡, and determines how rapidly the reaction. This is asking you to draw a potential energy diagram for an endothermic reaction. Lowering the activation energy of a reaction by a catalyst. This is because energy is taken in from the. The potential energy of the reaction is plotted along the vertical axis,. This graph compares potential energy diagrams. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. The energy level increases in an endothermic reaction. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses.
6.1 Activation energy (SL) YouTube
Endothermic Reaction Activation Energy Graph This is because energy is taken in from the. This is because energy is taken in from the. The potential energy of the reaction is plotted along the vertical axis,. Lowering the activation energy of a reaction by a catalyst. The energy changes that occur in reactions involving a multistep mechanism can be shown in a graph. It is possible to represent the endothermic reaction in the form of a graph called the energy diagram. This graph compares potential energy diagrams. This is asking you to draw a potential energy diagram for an endothermic reaction. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. The energy level increases in an endothermic reaction. The energy difference between reactants and the transition state is called the activation energy, δg ‡, and determines how rapidly the reaction. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product.
From www.chegg.com
Solved For a given exothermic reaction, how does the Endothermic Reaction Activation Energy Graph We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. This is asking you to draw a potential energy diagram for an endothermic reaction. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. The energy changes that occur in reactions involving a multistep mechanism can be. Endothermic Reaction Activation Energy Graph.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Activation Energy Graph The energy level increases in an endothermic reaction. This is because energy is taken in from the. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. The potential energy of the reaction is plotted along the vertical axis,. Recall that deltah_rxn, the enthalpy of reaction, is positive for. Endothermic Reaction Activation Energy Graph.
From www.dreamstime.com
Activation Energy Endothermic Reaction 2d Illustration Stock Endothermic Reaction Activation Energy Graph It is possible to represent the endothermic reaction in the form of a graph called the energy diagram. This is asking you to draw a potential energy diagram for an endothermic reaction. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. The energy level increases in an endothermic reaction. The energy difference between reactants and the. Endothermic Reaction Activation Energy Graph.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Activation Energy Graph The potential energy of the reaction is plotted along the vertical axis,. This is asking you to draw a potential energy diagram for an endothermic reaction. The energy difference between reactants and the transition state is called the activation energy, δg ‡, and determines how rapidly the reaction. This is because energy is taken in from the. Recall that deltah_rxn,. Endothermic Reaction Activation Energy Graph.
From www.coursehero.com
[Solved] /. Is this an endothermic or exothermic graph? Energy Diagram Endothermic Reaction Activation Energy Graph Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. The energy level increases in an endothermic reaction. This graph compares potential energy diagrams. It is possible to represent the endothermic reaction in the form of a graph called the energy diagram. We can graph the energy of a. Endothermic Reaction Activation Energy Graph.
From robhosking.com
10+ Endothermic Energy Diagram Robhosking Diagram Endothermic Reaction Activation Energy Graph The energy level increases in an endothermic reaction. It is possible to represent the endothermic reaction in the form of a graph called the energy diagram. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Lowering the activation energy of a reaction by a catalyst. The potential energy of. Endothermic Reaction Activation Energy Graph.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Endothermic Reaction Activation Energy Graph This is asking you to draw a potential energy diagram for an endothermic reaction. Lowering the activation energy of a reaction by a catalyst. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. The energy level increases in an endothermic reaction. This graph compares potential energy diagrams. Endothermic and. Endothermic Reaction Activation Energy Graph.
From professional.patrickneyman.com
Activation Energy Equation Endothermic Reaction Activation Energy Graph Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Lowering the activation energy of a reaction by a catalyst. This is because energy is taken in from the. The energy difference between reactants and the transition state is called the activation energy, δg ‡, and determines how rapidly. Endothermic Reaction Activation Energy Graph.
From www.studyorgo.com
Energy Diagram Module Series Part Two Gibbs Free Energy and Spontaneity Endothermic Reaction Activation Energy Graph This is because energy is taken in from the. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. The energy changes that occur in reactions involving a multistep mechanism can be shown in a graph. The energy level increases in an endothermic reaction. This graph compares potential energy diagrams.. Endothermic Reaction Activation Energy Graph.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction Activation Energy Graph The potential energy of the reaction is plotted along the vertical axis,. The energy difference between reactants and the transition state is called the activation energy, δg ‡, and determines how rapidly the reaction. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. Lowering the activation energy of a reaction by a catalyst. This is asking. Endothermic Reaction Activation Energy Graph.
From ar.inspiredpencil.com
Exothermic Reaction Graph Endothermic Reaction Activation Energy Graph This graph compares potential energy diagrams. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. This is because energy is taken in from the. The energy changes that occur in reactions involving a multistep mechanism can be shown in a graph. Lowering the activation energy of a reaction by a catalyst. The energy level increases in. Endothermic Reaction Activation Energy Graph.
From www.youtube.com
Endothermic Vs. Exothermic Reaction Graphs YouTube Endothermic Reaction Activation Energy Graph The energy difference between reactants and the transition state is called the activation energy, δg ‡, and determines how rapidly the reaction. The energy changes that occur in reactions involving a multistep mechanism can be shown in a graph. This is because energy is taken in from the. Lowering the activation energy of a reaction by a catalyst. This is. Endothermic Reaction Activation Energy Graph.
From www.varsitytutors.com
Reaction Coordinate Diagrams College Chemistry Endothermic Reaction Activation Energy Graph The energy changes that occur in reactions involving a multistep mechanism can be shown in a graph. It is possible to represent the endothermic reaction in the form of a graph called the energy diagram. This is asking you to draw a potential energy diagram for an endothermic reaction. Lowering the activation energy of a reaction by a catalyst. This. Endothermic Reaction Activation Energy Graph.
From schematron.org
Endothermic Reaction Coordinate Diagram Wiring Diagram Pictures Endothermic Reaction Activation Energy Graph This is because energy is taken in from the. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. The energy level increases in an endothermic reaction. The potential energy of the reaction is plotted along the vertical axis,. It is possible to represent the endothermic reaction in the form. Endothermic Reaction Activation Energy Graph.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation ID239015 Endothermic Reaction Activation Energy Graph Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. This is asking you to draw a potential energy diagram for an endothermic reaction. The energy changes that occur in reactions involving a multistep mechanism can be shown in a graph. Lowering the activation energy of a reaction by a catalyst. We can graph the energy of. Endothermic Reaction Activation Energy Graph.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction Activation Energy Graph This is asking you to draw a potential energy diagram for an endothermic reaction. The energy difference between reactants and the transition state is called the activation energy, δg ‡, and determines how rapidly the reaction. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Lowering the activation energy. Endothermic Reaction Activation Energy Graph.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Endothermic Reaction Activation Energy Graph The potential energy of the reaction is plotted along the vertical axis,. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Lowering the activation energy of a reaction by a catalyst. The energy changes that occur in reactions involving a multistep mechanism can be shown in a graph.. Endothermic Reaction Activation Energy Graph.
From manualpartsynaxis123.z13.web.core.windows.net
Exothermic And Endothermic Energy Diagrams Endothermic Reaction Activation Energy Graph Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Lowering the activation energy of a reaction by a catalyst. The energy difference between reactants and the transition state is called the activation energy, δg ‡, and. Endothermic Reaction Activation Energy Graph.
From byjus.com
24. What is the Minimum activation energy required for endothermic and Endothermic Reaction Activation Energy Graph The energy changes that occur in reactions involving a multistep mechanism can be shown in a graph. This is asking you to draw a potential energy diagram for an endothermic reaction. It is possible to represent the endothermic reaction in the form of a graph called the energy diagram. We can graph the energy of a reaction by plotting the. Endothermic Reaction Activation Energy Graph.
From www.reddit.com
Why does enthalpy of reaction with temperature change by Kirchoff's Law Endothermic Reaction Activation Energy Graph It is possible to represent the endothermic reaction in the form of a graph called the energy diagram. This is asking you to draw a potential energy diagram for an endothermic reaction. Lowering the activation energy of a reaction by a catalyst. The energy changes that occur in reactions involving a multistep mechanism can be shown in a graph. This. Endothermic Reaction Activation Energy Graph.
From drivenheisenberg.blogspot.com
What Is The Activation Energy For The Reaction In This Energy Diagram Endothermic Reaction Activation Energy Graph The energy level increases in an endothermic reaction. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. The potential energy of the reaction is plotted along the vertical axis,. The energy difference between reactants and the transition state is called the activation energy, δg ‡, and determines how rapidly the reaction. It is possible to represent. Endothermic Reaction Activation Energy Graph.
From www.youtube.com
6.1 Activation energy (SL) YouTube Endothermic Reaction Activation Energy Graph The energy level increases in an endothermic reaction. The energy difference between reactants and the transition state is called the activation energy, δg ‡, and determines how rapidly the reaction. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the. Endothermic Reaction Activation Energy Graph.
From socratic.org
What are activation energies? Socratic Endothermic Reaction Activation Energy Graph We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. The energy difference between reactants and the transition state is called the activation energy, δg ‡, and determines how rapidly the reaction. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. The energy changes that occur. Endothermic Reaction Activation Energy Graph.
From blogs.glowscotland.org.uk
Activation Energy Higher Chemistry Unit 1 Endothermic Reaction Activation Energy Graph This graph compares potential energy diagrams. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. The energy difference between reactants and the transition state is called the activation energy, δg ‡, and determines how rapidly the reaction. The potential energy of the reaction is plotted along the vertical. Endothermic Reaction Activation Energy Graph.
From stock.adobe.com
activation energy endothermic reaction diagram vector de Stock Adobe Endothermic Reaction Activation Energy Graph This is asking you to draw a potential energy diagram for an endothermic reaction. The energy changes that occur in reactions involving a multistep mechanism can be shown in a graph. It is possible to represent the endothermic reaction in the form of a graph called the energy diagram. This graph compares potential energy diagrams. The energy level increases in. Endothermic Reaction Activation Energy Graph.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction Activation Energy Graph This graph compares potential energy diagrams. Lowering the activation energy of a reaction by a catalyst. The potential energy of the reaction is plotted along the vertical axis,. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. The energy difference between reactants and the transition state is called. Endothermic Reaction Activation Energy Graph.
From www.youtube.com
Endothermic and Exothermic Reactions With Potential Energy Diagrams Endothermic Reaction Activation Energy Graph We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. The energy level increases in an endothermic reaction. This is asking you to draw a potential energy diagram for an endothermic reaction. The energy difference between reactants. Endothermic Reaction Activation Energy Graph.
From www.workmanagementsolutions.com.au
An Analogy The Activation Energy and Catalysis in Business Operations Endothermic Reaction Activation Energy Graph The potential energy of the reaction is plotted along the vertical axis,. The energy level increases in an endothermic reaction. The energy changes that occur in reactions involving a multistep mechanism can be shown in a graph. Lowering the activation energy of a reaction by a catalyst. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e.. Endothermic Reaction Activation Energy Graph.
From www.expii.com
Energy Diagram — Overview & Parts Expii Endothermic Reaction Activation Energy Graph Lowering the activation energy of a reaction by a catalyst. This is asking you to draw a potential energy diagram for an endothermic reaction. This is because energy is taken in from the. It is possible to represent the endothermic reaction in the form of a graph called the energy diagram. This graph compares potential energy diagrams. The energy level. Endothermic Reaction Activation Energy Graph.
From www.doubtnut.com
For an endothermic reaction energy of activation is E(a) and enthlpy o Endothermic Reaction Activation Energy Graph This is asking you to draw a potential energy diagram for an endothermic reaction. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. The energy changes that occur in reactions involving a multistep mechanism can be shown in a graph. The potential energy of the reaction is plotted. Endothermic Reaction Activation Energy Graph.
From mungfali.com
Exothermic Reaction Energy Graph Endothermic Reaction Activation Energy Graph Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. This graph compares potential energy diagrams. The potential energy of the reaction is plotted along the vertical axis,. This is because energy is taken in from. Endothermic Reaction Activation Energy Graph.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction Activation Energy Graph Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. The energy difference between reactants and the transition state is called the activation energy, δg ‡, and determines how. Endothermic Reaction Activation Energy Graph.
From ar.inspiredpencil.com
Exothermic Reaction Diagram Endothermic Reaction Activation Energy Graph We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. This is because energy is taken in from the. The energy difference between reactants and the transition state is called the activation energy, δg ‡, and determines how rapidly the reaction. The energy level increases in an endothermic reaction. This. Endothermic Reaction Activation Energy Graph.
From stock.adobe.com
endothermic reaction graph in chemistry Stock Vector Adobe Stock Endothermic Reaction Activation Energy Graph This graph compares potential energy diagrams. Recall that deltah_rxn, the enthalpy of reaction, is positive for endothermic reactions, i.e. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. The energy changes that occur in reactions involving a multistep mechanism can be shown in a graph. The energy difference between. Endothermic Reaction Activation Energy Graph.
From www.savemyexams.co.uk
Enthalpy Change & Activation Energy (5.1.2) CIE IGCSE Chemistry Endothermic Reaction Activation Energy Graph This is because energy is taken in from the. The energy changes that occur in reactions involving a multistep mechanism can be shown in a graph. We can graph the energy of a reaction by plotting the potential energy of the system as the reaction progresses. This is asking you to draw a potential energy diagram for an endothermic reaction.. Endothermic Reaction Activation Energy Graph.