Laboratory Method Of Preparation Of Nitric Acid . Preparation of nitric acid in the laboratory explained. Industrial preparation of nitric acid by ostwald's method.this video is about: Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature below 200 ℃. A 70% (w/w) nitric acid can be purchased from several commercial suppliers. Fall in love with chemistry! It is a clear colorless liquid and can be diluted to prepare. Hno 3, it is treated with conc. The simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or sodium nitrate). Preparation of 98% nitric acid : H 2 so 4, which acts as dehydrating agent. Understanding the different methods of. In this reaction, the salt of the more volatile acid is. For the preparation of conc. Preparation of nitric acid in laboratory.
from www.doubtnut.com
Hno 3, it is treated with conc. Industrial preparation of nitric acid by ostwald's method.this video is about: For the preparation of conc. H 2 so 4, which acts as dehydrating agent. The simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or sodium nitrate). Understanding the different methods of. Fall in love with chemistry! Preparation of nitric acid in laboratory. In this reaction, the salt of the more volatile acid is. Preparation of nitric acid in the laboratory explained.
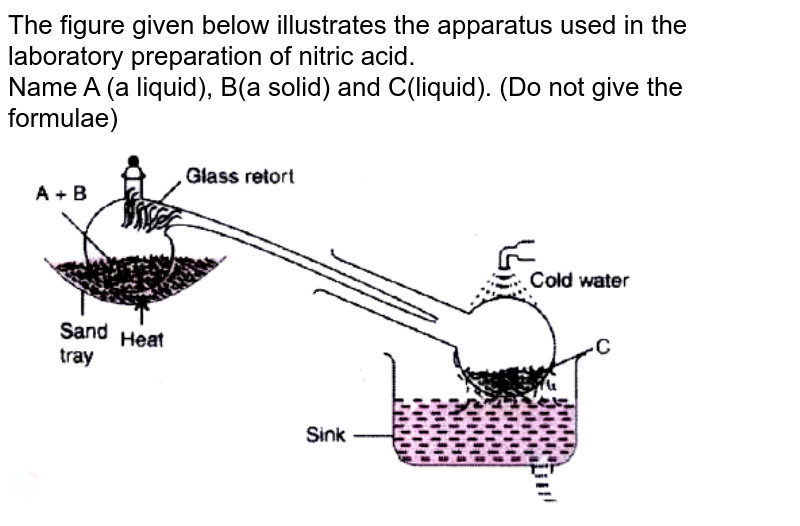
In the laboratory preparation of nitric acid Name the reactants A
Laboratory Method Of Preparation Of Nitric Acid Understanding the different methods of. A 70% (w/w) nitric acid can be purchased from several commercial suppliers. In this reaction, the salt of the more volatile acid is. Preparation of nitric acid in laboratory. Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature below 200 ℃. For the preparation of conc. Preparation of 98% nitric acid : The simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or sodium nitrate). Industrial preparation of nitric acid by ostwald's method.this video is about: Preparation of nitric acid in the laboratory explained. H 2 so 4, which acts as dehydrating agent. Hno 3, it is treated with conc. It is a clear colorless liquid and can be diluted to prepare. Fall in love with chemistry! Understanding the different methods of.
From www.tradeindia.com
Nitric Acid In Ludhiana, Nitric Acid Dealers & Traders In Ludhiana, Punjab Laboratory Method Of Preparation Of Nitric Acid Hno 3, it is treated with conc. Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature below 200 ℃. Preparation of nitric acid in laboratory. The simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium. Laboratory Method Of Preparation Of Nitric Acid.
From icsechemistry16.blogspot.com
Nitric acid lab preparation precautions Laboratory Method Of Preparation Of Nitric Acid A 70% (w/w) nitric acid can be purchased from several commercial suppliers. Understanding the different methods of. It is a clear colorless liquid and can be diluted to prepare. Industrial preparation of nitric acid by ostwald's method.this video is about: For the preparation of conc. Preparation of nitric acid in laboratory. In this reaction, the salt of the more volatile. Laboratory Method Of Preparation Of Nitric Acid.
From www.youtube.com
Preparation of nitric acid from class 12 nitric acid preparation and Laboratory Method Of Preparation Of Nitric Acid A 70% (w/w) nitric acid can be purchased from several commercial suppliers. Preparation of nitric acid in the laboratory explained. Industrial preparation of nitric acid by ostwald's method.this video is about: H 2 so 4, which acts as dehydrating agent. Preparation of 98% nitric acid : For the preparation of conc. Preparation of nitric acid in laboratory. In this reaction,. Laboratory Method Of Preparation Of Nitric Acid.
From www.researchgate.net
Different types of nitric acid synthesis process, (a) Birkeland & Eyde Laboratory Method Of Preparation Of Nitric Acid Hno 3, it is treated with conc. For the preparation of conc. The simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or sodium nitrate). Fall in love with chemistry! Understanding the different methods of. Preparation of nitric acid in laboratory. H 2 so 4,. Laboratory Method Of Preparation Of Nitric Acid.
From www.youtube.com
Laboratory Preparation of Nitric Acid YouTube Laboratory Method Of Preparation Of Nitric Acid Preparation of nitric acid in laboratory. For the preparation of conc. Understanding the different methods of. It is a clear colorless liquid and can be diluted to prepare. Industrial preparation of nitric acid by ostwald's method.this video is about: Fall in love with chemistry! Preparation of nitric acid in the laboratory explained. H 2 so 4, which acts as dehydrating. Laboratory Method Of Preparation Of Nitric Acid.
From www.youtube.com
Preparation of Nitric Acid in Laboratory, Chemistry Lecture Sabaq.pk Laboratory Method Of Preparation Of Nitric Acid A 70% (w/w) nitric acid can be purchased from several commercial suppliers. H 2 so 4, which acts as dehydrating agent. For the preparation of conc. In this reaction, the salt of the more volatile acid is. The simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt. Laboratory Method Of Preparation Of Nitric Acid.
From www.youtube.com
How to prepare dilute solution of nitric acid HNO3 in laboratory YouTube Laboratory Method Of Preparation Of Nitric Acid Preparation of nitric acid in the laboratory explained. It is a clear colorless liquid and can be diluted to prepare. Preparation of nitric acid in laboratory. Preparation of 98% nitric acid : H 2 so 4, which acts as dehydrating agent. Hno 3, it is treated with conc. Understanding the different methods of. The simplest and most used preparation of. Laboratory Method Of Preparation Of Nitric Acid.
From www.slideserve.com
PPT LECTURE (10) NITRIC ACID PRODUCTION 1INTRODUCTION PowerPoint Laboratory Method Of Preparation Of Nitric Acid Understanding the different methods of. For the preparation of conc. Preparation of nitric acid in laboratory. Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature below 200 ℃. Preparation of 98% nitric acid : Hno 3, it is treated with conc. H 2 so 4, which acts as dehydrating agent.. Laboratory Method Of Preparation Of Nitric Acid.
From stock.adobe.com
Vector illustration of nitric acid production. Nitric acid synthesis Laboratory Method Of Preparation Of Nitric Acid Preparation of nitric acid in laboratory. It is a clear colorless liquid and can be diluted to prepare. A 70% (w/w) nitric acid can be purchased from several commercial suppliers. Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature below 200 ℃. Preparation of 98% nitric acid : The simplest. Laboratory Method Of Preparation Of Nitric Acid.
From www.shutterstock.com
Nitric Acid Bottle Chemical Laboratory Industry Stock Photo 2161868079 Laboratory Method Of Preparation Of Nitric Acid For the preparation of conc. Hno 3, it is treated with conc. Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature below 200 ℃. Preparation of 98% nitric acid : Industrial preparation of nitric acid by ostwald's method.this video is about: H 2 so 4, which acts as dehydrating agent.. Laboratory Method Of Preparation Of Nitric Acid.
From www.researchgate.net
Process flow diagram of nitric acid synthesis plant. Download Laboratory Method Of Preparation Of Nitric Acid Industrial preparation of nitric acid by ostwald's method.this video is about: Fall in love with chemistry! It is a clear colorless liquid and can be diluted to prepare. Preparation of 98% nitric acid : For the preparation of conc. The simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry. Laboratory Method Of Preparation Of Nitric Acid.
From www.vecteezy.com
Preparation of Nitric Acidin in laboratory 23587363 Vector Art at Vecteezy Laboratory Method Of Preparation Of Nitric Acid Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature below 200 ℃. Preparation of nitric acid in laboratory. Understanding the different methods of. For the preparation of conc. Fall in love with chemistry! Preparation of 98% nitric acid : In this reaction, the salt of the more volatile acid is.. Laboratory Method Of Preparation Of Nitric Acid.
From brainly.in
explain the ostwald method of prepration of nitric acid with the help Laboratory Method Of Preparation Of Nitric Acid For the preparation of conc. H 2 so 4, which acts as dehydrating agent. A 70% (w/w) nitric acid can be purchased from several commercial suppliers. Understanding the different methods of. Preparation of 98% nitric acid : The simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt. Laboratory Method Of Preparation Of Nitric Acid.
From iqclasses.in
Class 10 ICSE Chemistry Mostlikely QuestionBank Chapter Nitric Acid Laboratory Method Of Preparation Of Nitric Acid H 2 so 4, which acts as dehydrating agent. Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature below 200 ℃. Understanding the different methods of. Preparation of 98% nitric acid : Preparation of nitric acid in laboratory. A 70% (w/w) nitric acid can be purchased from several commercial suppliers.. Laboratory Method Of Preparation Of Nitric Acid.
From www.youtube.com
10ALab preparation of Nitric Acid YouTube Laboratory Method Of Preparation Of Nitric Acid A 70% (w/w) nitric acid can be purchased from several commercial suppliers. Industrial preparation of nitric acid by ostwald's method.this video is about: Preparation of nitric acid in laboratory. Preparation of 98% nitric acid : For the preparation of conc. Understanding the different methods of. Fall in love with chemistry! H 2 so 4, which acts as dehydrating agent. Hno. Laboratory Method Of Preparation Of Nitric Acid.
From www.youtube.com
Ostwald Method for the Preparation of Nitric Acid Class 12 Chemistry Laboratory Method Of Preparation Of Nitric Acid In this reaction, the salt of the more volatile acid is. A 70% (w/w) nitric acid can be purchased from several commercial suppliers. It is a clear colorless liquid and can be diluted to prepare. Preparation of nitric acid in laboratory. Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature. Laboratory Method Of Preparation Of Nitric Acid.
From www.youtube.com
Laboratory Preparation of Nitric AcidNITRIC ACID Class10 ICSE Laboratory Method Of Preparation Of Nitric Acid It is a clear colorless liquid and can be diluted to prepare. Preparation of nitric acid in the laboratory explained. Understanding the different methods of. For the preparation of conc. Hno 3, it is treated with conc. Preparation of nitric acid in laboratory. Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at. Laboratory Method Of Preparation Of Nitric Acid.
From www.doubtnut.com
In the laboratory preparation of nitric acid Name the reactants A Laboratory Method Of Preparation Of Nitric Acid For the preparation of conc. Hno 3, it is treated with conc. The simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or sodium nitrate). Fall in love with chemistry! It is a clear colorless liquid and can be diluted to prepare. In this reaction,. Laboratory Method Of Preparation Of Nitric Acid.
From www.youtube.com
Nitric Acid & it's Preparation Method YouTube Laboratory Method Of Preparation Of Nitric Acid Hno 3, it is treated with conc. Preparation of 98% nitric acid : A 70% (w/w) nitric acid can be purchased from several commercial suppliers. Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature below 200 ℃. H 2 so 4, which acts as dehydrating agent. Preparation of nitric acid. Laboratory Method Of Preparation Of Nitric Acid.
From www.youtube.com
Preparation of Nitric Acid in Laboratory Reaction HNO3 in the Lab Laboratory Method Of Preparation Of Nitric Acid Industrial preparation of nitric acid by ostwald's method.this video is about: Preparation of 98% nitric acid : The simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or sodium nitrate). Fall in love with chemistry! It is a clear colorless liquid and can be diluted. Laboratory Method Of Preparation Of Nitric Acid.
From owlcation.com
3 Ways to Prepare Nitric Acid Owlcation Laboratory Method Of Preparation Of Nitric Acid Preparation of nitric acid in laboratory. Hno 3, it is treated with conc. Industrial preparation of nitric acid by ostwald's method.this video is about: Preparation of 98% nitric acid : Understanding the different methods of. Preparation of nitric acid in the laboratory explained. H 2 so 4, which acts as dehydrating agent. In this reaction, the salt of the more. Laboratory Method Of Preparation Of Nitric Acid.
From solutionpharmacy.in
How is nitric acid prepared? Solution Parmacy Laboratory Method Of Preparation Of Nitric Acid For the preparation of conc. H 2 so 4, which acts as dehydrating agent. Fall in love with chemistry! Industrial preparation of nitric acid by ostwald's method.this video is about: It is a clear colorless liquid and can be diluted to prepare. Preparation of nitric acid in laboratory. Understanding the different methods of. In this reaction, the salt of the. Laboratory Method Of Preparation Of Nitric Acid.
From owlcation.com
3 Ways to Prepare Nitric Acid Owlcation Laboratory Method Of Preparation Of Nitric Acid A 70% (w/w) nitric acid can be purchased from several commercial suppliers. Preparation of nitric acid in laboratory. Preparation of nitric acid in the laboratory explained. Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature below 200 ℃. Hno 3, it is treated with conc. In this reaction, the salt. Laboratory Method Of Preparation Of Nitric Acid.
From sajhanotes.com
Nitric Acid NEB Grade 11 Notes Chemistry Sajha Notes Laboratory Method Of Preparation Of Nitric Acid It is a clear colorless liquid and can be diluted to prepare. Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature below 200 ℃. Preparation of nitric acid in laboratory. Fall in love with chemistry! Hno 3, it is treated with conc. The simplest and most used preparation of nitric. Laboratory Method Of Preparation Of Nitric Acid.
From www.youtube.com
Concentrated nitric acid used in laboratory work is 68 nitric acid by Laboratory Method Of Preparation Of Nitric Acid For the preparation of conc. A 70% (w/w) nitric acid can be purchased from several commercial suppliers. In this reaction, the salt of the more volatile acid is. Understanding the different methods of. Preparation of 98% nitric acid : Industrial preparation of nitric acid by ostwald's method.this video is about: Fall in love with chemistry! Nitric acid is prepared in. Laboratory Method Of Preparation Of Nitric Acid.
From www.doubtnut.com
Explain the following In the laboratory preparation of nitric ac Laboratory Method Of Preparation Of Nitric Acid H 2 so 4, which acts as dehydrating agent. Hno 3, it is treated with conc. Understanding the different methods of. It is a clear colorless liquid and can be diluted to prepare. Preparation of 98% nitric acid : Preparation of nitric acid in laboratory. Preparation of nitric acid in the laboratory explained. For the preparation of conc. A 70%. Laboratory Method Of Preparation Of Nitric Acid.
From www.alamy.com
Laboratory preparation of nitric (V) acid fully labeled diagram Stock Laboratory Method Of Preparation Of Nitric Acid Preparation of 98% nitric acid : Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature below 200 ℃. The simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or sodium nitrate). Fall in love with. Laboratory Method Of Preparation Of Nitric Acid.
From www.youtube.com
Preparation of Nitric acid by Ostwald method YouTube Laboratory Method Of Preparation Of Nitric Acid Preparation of 98% nitric acid : In this reaction, the salt of the more volatile acid is. It is a clear colorless liquid and can be diluted to prepare. Understanding the different methods of. A 70% (w/w) nitric acid can be purchased from several commercial suppliers. Preparation of nitric acid in the laboratory explained. Industrial preparation of nitric acid by. Laboratory Method Of Preparation Of Nitric Acid.
From www.youtube.com
Manufacture of Nitric Acid by ammonia oxidation process nitric acid Laboratory Method Of Preparation Of Nitric Acid Understanding the different methods of. For the preparation of conc. Industrial preparation of nitric acid by ostwald's method.this video is about: Preparation of nitric acid in the laboratory explained. Preparation of nitric acid in laboratory. Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature below 200 ℃. Fall in love. Laboratory Method Of Preparation Of Nitric Acid.
From www.doubtnut.com
Explain the following In the laboratory preparation of nitric ac Laboratory Method Of Preparation Of Nitric Acid For the preparation of conc. The simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or sodium nitrate). A 70% (w/w) nitric acid can be purchased from several commercial suppliers. Preparation of nitric acid in the laboratory explained. Fall in love with chemistry! Understanding the. Laboratory Method Of Preparation Of Nitric Acid.
From www.youtube.com
Preparation of 1N Nitric Acid YouTube Laboratory Method Of Preparation Of Nitric Acid In this reaction, the salt of the more volatile acid is. A 70% (w/w) nitric acid can be purchased from several commercial suppliers. Preparation of nitric acid in the laboratory explained. The simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or sodium nitrate). Fall. Laboratory Method Of Preparation Of Nitric Acid.
From www.slideserve.com
PPT Nitric Acid (HNO3) PowerPoint Presentation ID2478951 Laboratory Method Of Preparation Of Nitric Acid It is a clear colorless liquid and can be diluted to prepare. In this reaction, the salt of the more volatile acid is. Preparation of 98% nitric acid : Understanding the different methods of. Hno 3, it is treated with conc. H 2 so 4, which acts as dehydrating agent. Industrial preparation of nitric acid by ostwald's method.this video is. Laboratory Method Of Preparation Of Nitric Acid.
From www.youtube.com
Laboratory Preparation of Nitric Acid Part 1 ICSE Class 10 Laboratory Method Of Preparation Of Nitric Acid Understanding the different methods of. Preparation of nitric acid in laboratory. Preparation of 98% nitric acid : Hno 3, it is treated with conc. A 70% (w/w) nitric acid can be purchased from several commercial suppliers. Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature below 200 ℃. For the. Laboratory Method Of Preparation Of Nitric Acid.
From www.dbioscharts.com
CH 900 PREPARATION OF NITRIC ACID Dbios Charts Laboratory Method Of Preparation Of Nitric Acid The simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or sodium nitrate). For the preparation of conc. H 2 so 4, which acts as dehydrating agent. Hno 3, it is treated with conc. A 70% (w/w) nitric acid can be purchased from several commercial. Laboratory Method Of Preparation Of Nitric Acid.
From www.youtube.com
LABORATORY PREPARATION OF NITRIC ACID YouTube Laboratory Method Of Preparation Of Nitric Acid Nitric acid is prepared in the laboratory by mixing alkaline nitrate salt with concentrated nitric acid at a temperature below 200 ℃. Understanding the different methods of. It is a clear colorless liquid and can be diluted to prepare. Hno 3, it is treated with conc. The simplest and most used preparation of nitric acid in the lab is by. Laboratory Method Of Preparation Of Nitric Acid.