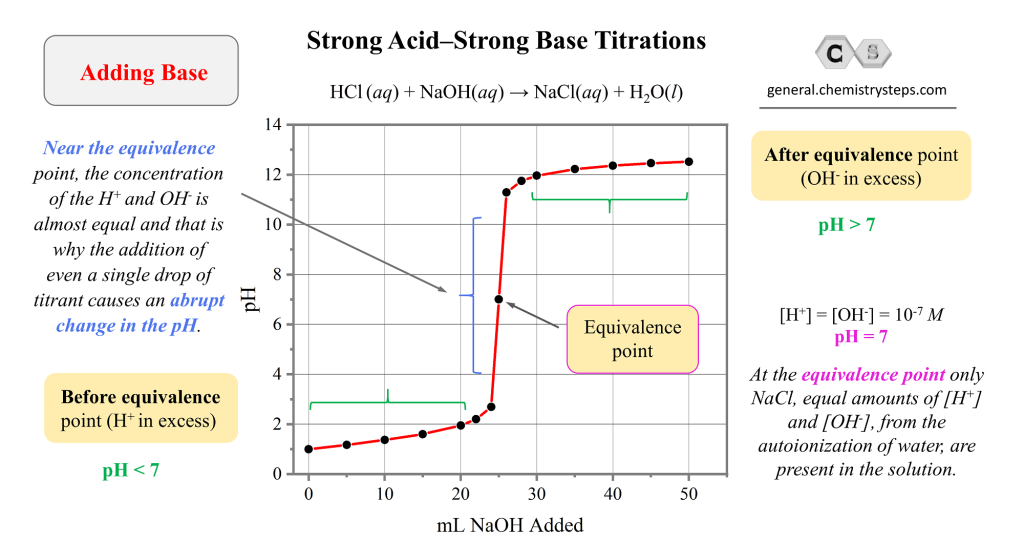
Titration Curve Number . A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point of a titration is the point at which an indicator changes colour at a. The figure below shows two different examples of a strong acid. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The volume of the titrant added. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. The equivalence point of a titration.
from general.chemistrysteps.com
The figure below shows two different examples of a strong acid. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. The end point of a titration is the point at which an indicator changes colour at a. A titration curve is a graphical representation of the ph of a solution during a titration. The volume of the titrant added. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The equivalence point of a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it.
Strong AcidStrong Base Titrations Chemistry Steps
Titration Curve Number The figure below shows two different examples of a strong acid. The end point of a titration is the point at which an indicator changes colour at a. The volume of the titrant added. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The figure below shows two different examples of a strong acid. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Number The figure below shows two different examples of a strong acid. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. The equivalence point of a titration.. Titration Curve Number.
From www.chegg.com
Solved Use the titration curve shown below to answer Titration Curve Number The equivalence point of a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The figure below shows two different examples of a strong acid. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction.. Titration Curve Number.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curve Number A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point of a titration is the point at which an indicator changes colour at a. Titrations are often recorded. Titration Curve Number.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Number Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The figure below shows two different examples of a strong acid. The volume of the titrant added. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is. Titration Curve Number.
From ar.inspiredpencil.com
Titration Curve Labeled Titration Curve Number Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The figure below shows two different examples of a strong acid. The equivalence point of a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. Titration Curve Number.
From mungfali.com
Endpoint Titration Curve Titration Curve Number The equivalence point of a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The figure below shows two different examples of a strong acid. The volume of the titrant added. A titration curve is a graphical representation of the ph of a solution during. Titration Curve Number.
From www.animalia-life.club
Titration Curve Amino Acid Titration Curve Number The end point of a titration is the point at which an indicator changes colour at a. The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong acid. Titrations are often recorded on graphs called titration curves, which. Titration Curve Number.
From www.chegg.com
The graphs show a titration curve for the amino acid Titration Curve Number Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. A titration curve is a graphical representation of the ph of a solution during. Titration Curve Number.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Curve Number A titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at which an indicator changes colour at a. The equivalence point of a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable. Titration Curve Number.
From www.writework.com
Titration of amino acids WriteWork Titration Curve Number The volume of the titrant added. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. The end point of a titration is the point at which. Titration Curve Number.
From www.chegg.com
Solved The titration curve below shows the results of the Titration Curve Number Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. The volume of the titrant added. The figure below shows two different examples of a strong acid.. Titration Curve Number.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Number Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The figure below shows two different examples of a strong acid. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. The volume of the titrant added.. Titration Curve Number.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve Number The end point of a titration is the point at which an indicator changes colour at a. The volume of the titrant added. The figure below shows two different examples of a strong acid. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titrations are often recorded on graphs. Titration Curve Number.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Titration Curve Number Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The equivalence point of a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titration is a widely used technique to measure. Titration Curve Number.
From www.savemyexams.com
pH Titration Curves OCR A Level Chemistry Revision Notes 2017 Titration Curve Number A titration curve is a graphical representation of the ph of a solution during a titration. The volume of the titrant added. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The figure below shows two different examples of a strong acid. The equivalence point of. Titration Curve Number.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Titration Curve Number The end point of a titration is the point at which an indicator changes colour at a. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of. Titration Curve Number.
From slidetodoc.com
Acid Base Titrations Titration Curve A titration curve Titration Curve Number The equivalence point of a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The figure below shows two different examples of. Titration Curve Number.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Number Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The equivalence point of a titration. The volume of the titrant added. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic. Titration Curve Number.
From www.chegg.com
Solved Titration curve 14 12 10 6 4 0 0 10 20 30 40 50 60 70 Titration Curve Number The end point of a titration is the point at which an indicator changes colour at a. A titration curve is a graphical representation of the ph of a solution during a titration. The volume of the titrant added. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable. Titration Curve Number.
From www.youtube.com
Titration Curves for High School Chemistry YouTube Titration Curve Number Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The volume of the titrant added. The figure below shows two different examples of a strong acid. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is. Titration Curve Number.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Number The equivalence point of a titration. The end point of a titration is the point at which an indicator changes colour at a. A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong acid. Titrations are often recorded on graphs called titration curves, which. Titration Curve Number.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Titration Curve Number The end point of a titration is the point at which an indicator changes colour at a. The figure below shows two different examples of a strong acid. The equivalence point of a titration. The volume of the titrant added. A titration curve is a graphical representation of the ph of a solution during a titration. Titrations are often recorded. Titration Curve Number.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Number The equivalence point of a titration. The volume of the titrant added. A titration curve is a graphical representation of the ph of a solution during a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The end point of a titration is the point. Titration Curve Number.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Curve Number The volume of the titrant added. The figure below shows two different examples of a strong acid. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how. Titration Curve Number.
From www.doubtnut.com
Exercise from NARENDER AVASTHI CHEMISTRY Chapter 6 IONIC EEQUILIBRIUM Titration Curve Number The volume of the titrant added. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a graphical representation of the ph of a solution during a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of. Titration Curve Number.
From www.numerade.com
SOLVED Match the provided labels to the appropriate point on the Titration Curve Number The equivalence point of a titration. The end point of a titration is the point at which an indicator changes colour at a. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The figure below shows two different examples of a strong acid. A titration curve. Titration Curve Number.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curve Number A titration curve is a graphical representation of the ph of a solution during a titration. The equivalence point of a titration. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. Titration curves show how the ph of an acidic or basic solution changes as a. Titration Curve Number.
From www.chegg.com
Solved Here is a titration curve for 10.00 mL of an unknown Titration Curve Number Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point of a titration is the point at which an indicator changes colour at a. A titration curve is a graphical representation of the ph of a solution during a titration. Titrations are often recorded. Titration Curve Number.
From ar.inspiredpencil.com
Titration Curve Labeled Titration Curve Number Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point of a titration is the point at which an indicator changes colour at a. The figure below shows two different examples of a strong acid. Titrations are often recorded on graphs called titration curves,. Titration Curve Number.
From www.slideserve.com
PPT Acidity Constant by pH Titration Curves PowerPoint Presentation Titration Curve Number The end point of a titration is the point at which an indicator changes colour at a. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the. The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during. Titration Curve Number.
From socratic.org
Titration curve? Please help... Socratic Titration Curve Number The volume of the titrant added. The figure below shows two different examples of a strong acid. The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added. Titration Curve Number.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Curve Number Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The equivalence point of a titration. The end point of a titration is the point at which an indicator changes colour at a. Titration is a widely used technique to measure the amount of one substance present. Titration Curve Number.
From tophat.com
OpenStax Atoms First Chemistry 14.7 AcidBase Titrations Top Hat Titration Curve Number Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. The end point of a titration is the point at which an indicator changes colour at a. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it.. Titration Curve Number.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Number Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. The equivalence point of a titration. The volume of the titrant added. The figure below shows two different examples of a strong acid. Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant. Titration Curve Number.
From brainly.com
Please answer the following questions based on the titration curve 1 Titration Curve Number Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. The figure below shows two different examples of a strong acid. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The volume of the titrant added.. Titration Curve Number.