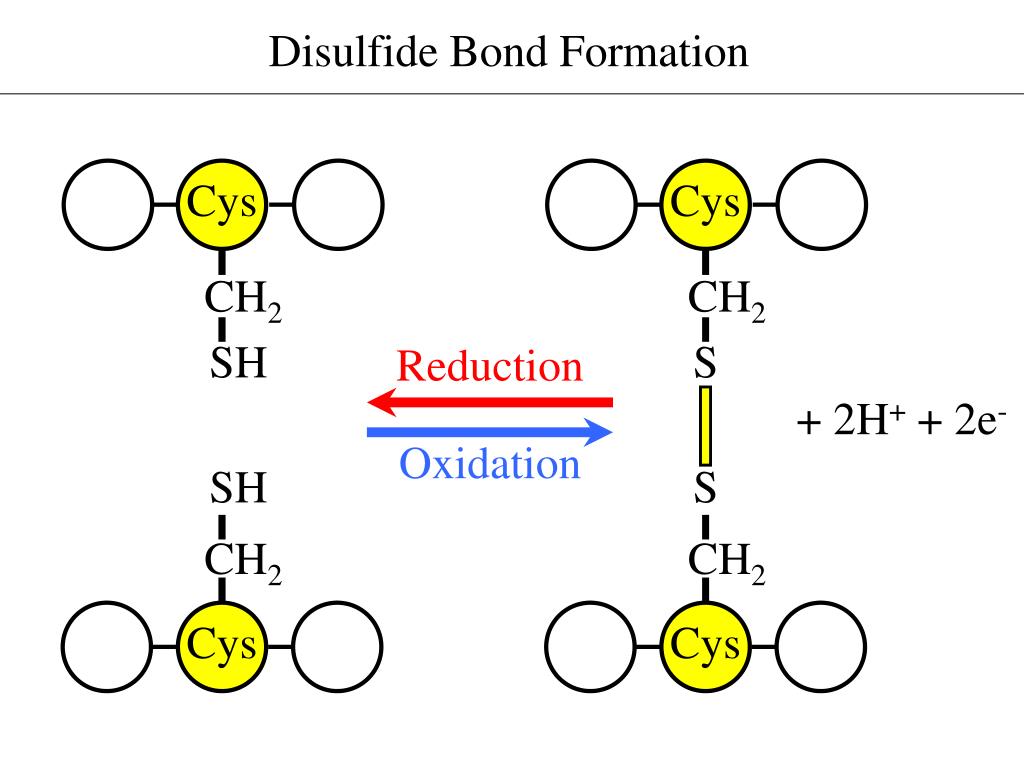
Disulfide Bond Protein Aggregation Reduction . In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. The disulfide rebridging strategy has. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. Intramolecular disulfide bonds are known to contribute to protein thermal stability.
from www.slideserve.com
In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. The disulfide rebridging strategy has. Intramolecular disulfide bonds are known to contribute to protein thermal stability. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with.
PPT Making the right connections Disulfide Bond Formation in the
Disulfide Bond Protein Aggregation Reduction The disulfide rebridging strategy has. The disulfide rebridging strategy has. In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. Intramolecular disulfide bonds are known to contribute to protein thermal stability.
From www.science.org
Disulfide Formation in the ER and Mitochondria Two Solutions to a Disulfide Bond Protein Aggregation Reduction Intramolecular disulfide bonds are known to contribute to protein thermal stability. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. The disulfide rebridging strategy has. In hirudin and some other. Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
The mechanisms of degradation of disulfide bonds in proteins upon Disulfide Bond Protein Aggregation Reduction In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. Intramolecular disulfide bonds are known to contribute to protein thermal stability. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with.. Disulfide Bond Protein Aggregation Reduction.
From www.pnas.org
Forcedependent chemical of disulfide bond reduction observed Disulfide Bond Protein Aggregation Reduction In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. Disulfide bonds play a key role in stabilizing protein structures, with. Disulfide Bond Protein Aggregation Reduction.
From www.science.org
An Engineered Pathway for the Formation of Protein Disulfide Bonds Disulfide Bond Protein Aggregation Reduction The disulfide rebridging strategy has. Intramolecular disulfide bonds are known to contribute to protein thermal stability. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. In hirudin and some other. Disulfide Bond Protein Aggregation Reduction.
From pubs.acs.org
Revisiting DisulfideYne and DisulfideDiazonium Reactions for Disulfide Bond Protein Aggregation Reduction Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. The disulfide rebridging strategy has. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. Intramolecular disulfide bonds are known to contribute to protein. Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
The disulfidebondforming pathways in the periplasm of E. coli. A Disulfide Bond Protein Aggregation Reduction Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. Intramolecular disulfide bonds are known to contribute to protein thermal stability. In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. The disulfide rebridging strategy has. In hirudin and some other. Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
Illustration of disulphide bond in a protein at various levels Disulfide Bond Protein Aggregation Reduction In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. Intramolecular disulfide bonds are known to contribute to protein thermal stability. The disulfide rebridging strategy has. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss. Disulfide Bond Protein Aggregation Reduction.
From www.mdpi.com
Molecules Free FullText PDIRegulated Disulfide Bond Formation in Disulfide Bond Protein Aggregation Reduction Intramolecular disulfide bonds are known to contribute to protein thermal stability. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. In a reduction. Disulfide Bond Protein Aggregation Reduction.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Disulfide bridge Disulfide Bond Protein Aggregation Reduction The disulfide rebridging strategy has. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. Intramolecular disulfide bonds are known to contribute to protein. Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
The mechanisms of degradation of disulfide bonds in proteins upon Disulfide Bond Protein Aggregation Reduction In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. Intramolecular disulfide bonds are known to contribute to protein thermal stability. In hirudin and some other proteins containing three disulfide bonds,. Disulfide Bond Protein Aggregation Reduction.
From royalsocietypublishing.org
Interleukin2 signalling is modulated by a labile disulfide bond in the Disulfide Bond Protein Aggregation Reduction Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. Intramolecular disulfide bonds are known to contribute to protein thermal stability. The disulfide rebridging. Disulfide Bond Protein Aggregation Reduction.
From www.slideserve.com
PPT Protein structure PowerPoint Presentation, free download ID9193673 Disulfide Bond Protein Aggregation Reduction Intramolecular disulfide bonds are known to contribute to protein thermal stability. The disulfide rebridging strategy has. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss. Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
Molecular mechanisms involved in Cuinduced aggregation of human Disulfide Bond Protein Aggregation Reduction The disulfide rebridging strategy has. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. Intramolecular disulfide bonds are known to contribute to protein thermal stability. In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. In hirudin and some other. Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
Reduction of disulfide bonds with DTT makes BRICHOS less effective as Disulfide Bond Protein Aggregation Reduction In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. The disulfide rebridging strategy has. Intramolecular disulfide bonds are known to contribute to protein. Disulfide Bond Protein Aggregation Reduction.
From www.mdpi.com
Molecules Free FullText PDIRegulated Disulfide Bond Formation in Disulfide Bond Protein Aggregation Reduction Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. Intramolecular disulfide bonds are known to contribute to protein thermal stability. The disulfide rebridging strategy has. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case). Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
Structure of the V3 domain disulfide bond and mechanism of cleavage. A Disulfide Bond Protein Aggregation Reduction Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. Intramolecular disulfide bonds are known to contribute to protein thermal stability. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. In a reduction. Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
(a) Dual functionalisation approach to rebridging disulfide bonds on Disulfide Bond Protein Aggregation Reduction In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. The disulfide rebridging strategy has. Intramolecular disulfide bonds are known to contribute to protein thermal stability. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n. Disulfide Bond Protein Aggregation Reduction.
From www.pnas.org
Mechanistic insights on the reduction of glutathione disulfide by Disulfide Bond Protein Aggregation Reduction Intramolecular disulfide bonds are known to contribute to protein thermal stability. In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with.. Disulfide Bond Protein Aggregation Reduction.
From www.slideserve.com
PPT Disulfide Bonds PowerPoint Presentation ID165240 Disulfide Bond Protein Aggregation Reduction The disulfide rebridging strategy has. Intramolecular disulfide bonds are known to contribute to protein thermal stability. In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n. Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
Mechanism of disulfide formation and isomerization in the bacterial Disulfide Bond Protein Aggregation Reduction In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. The disulfide rebridging strategy has. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. Intramolecular disulfide bonds are known to. Disulfide Bond Protein Aggregation Reduction.
From cshperspectives.cshlp.org
Disulfide Bond Formation in the Mammalian Endoplasmic Reticulum Disulfide Bond Protein Aggregation Reduction Intramolecular disulfide bonds are known to contribute to protein thermal stability. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol.. Disulfide Bond Protein Aggregation Reduction.
From www.pnas.org
Bacterial species exhibit diversity in their mechanisms and capacity Disulfide Bond Protein Aggregation Reduction In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. Intramolecular disulfide bonds are known to contribute to protein thermal stability. In hirudin and some other proteins containing three disulfide bonds,. Disulfide Bond Protein Aggregation Reduction.
From www.mdpi.com
Molecules Free FullText Revisiting the Formation of a Native Disulfide Bond Protein Aggregation Reduction In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. Intramolecular disulfide bonds are known to contribute to protein thermal stability. The disulfide rebridging strategy has. In hirudin and some other. Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
Thioldisulfide exchange reactions. The thioredoxin family proteins Disulfide Bond Protein Aggregation Reduction Intramolecular disulfide bonds are known to contribute to protein thermal stability. In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. The disulfide rebridging strategy has. In hirudin and some other. Disulfide Bond Protein Aggregation Reduction.
From www.wikidoc.org
Disulfide bond wikidoc Disulfide Bond Protein Aggregation Reduction In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. Disulfide bonds play a key role in stabilizing protein structures, with. Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
Schematic representation of the processes following disulfide bond Disulfide Bond Protein Aggregation Reduction In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. Intramolecular disulfide bonds are known to contribute to protein thermal stability. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. In a reduction. Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
Proposed reaction pathway that yields new disulfide crosslinked Disulfide Bond Protein Aggregation Reduction In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. The disulfide rebridging strategy has. In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. Disulfide bonds play a key role. Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
Reaction mechanism of disulfide reduction by thioredoxin. A schematic Disulfide Bond Protein Aggregation Reduction Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. The disulfide rebridging strategy has. Intramolecular disulfide bonds are known to contribute to protein thermal stability. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case). Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
Disulfidebridge chaining of domainswapped (DS) dimers in the natural Disulfide Bond Protein Aggregation Reduction The disulfide rebridging strategy has. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. Intramolecular disulfide bonds are known to contribute to protein thermal stability. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss. Disulfide Bond Protein Aggregation Reduction.
From www.slideserve.com
PPT Making the right connections Disulfide Bond Formation in the Disulfide Bond Protein Aggregation Reduction In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. Disulfide bonds play a key role in stabilizing protein structures, with. Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
Mechanisms of cleavage of allosteric disulfide bonds. Disulfide bond Disulfide Bond Protein Aggregation Reduction In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which. Disulfide Bond Protein Aggregation Reduction.
From www.cell.com
How Are Proteins Reduced in the Endoplasmic Reticulum? Trends in Disulfide Bond Protein Aggregation Reduction Intramolecular disulfide bonds are known to contribute to protein thermal stability. The disulfide rebridging strategy has. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide. Disulfide Bond Protein Aggregation Reduction.
From www.researchgate.net
The mechanisms of degradation of disulfide bonds in proteins upon Disulfide Bond Protein Aggregation Reduction The disulfide rebridging strategy has. In hirudin and some other proteins containing three disulfide bonds, the conformational folding of the 2s native species to 3s* (which is n in this case) competes with. Intramolecular disulfide bonds are known to contribute to protein thermal stability. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss. Disulfide Bond Protein Aggregation Reduction.
From www.jbc.org
Allosteric disulfides Sophisticated molecular structures enabling Disulfide Bond Protein Aggregation Reduction In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. Intramolecular disulfide bonds are known to contribute to protein thermal stability. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. The disulfide rebridging strategy has. In hirudin and some other. Disulfide Bond Protein Aggregation Reduction.
From www.mdpi.com
Mechanisms of Disulfide Bond Formation in Nascent Polypeptides Entering Disulfide Bond Protein Aggregation Reduction Intramolecular disulfide bonds are known to contribute to protein thermal stability. Disulfide bonds play a key role in stabilizing protein structures, with disruption strongly associated with loss of protein. In a reduction reaction, whereby pdi breaks down disulfide bonds in protein substrates, a substrate disulfide is reduced to the dithiol. The disulfide rebridging strategy has. In hirudin and some other. Disulfide Bond Protein Aggregation Reduction.