A Tank Contains Helium Gas At 490 Mmhg . First, convert the pressure of helium from mmhg to atm: A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520 mmhg. Here’s how to approach this question. The total pressure in the tank, which contains helium at 490 mmhg, nitrogen at 0.75 atm, and neon at 520 torr, is 2.1 atm. Mmhg and the partial pressures of. To convert mmhg to atm, use the. Convert the pressure of helium from mmhg to atm. (1 atm = 760 mmhg) A tank contains a mixture of helium, neon, and argon gases. 1 atm = 760 mmhg so, helium pressure in atm = 490 mmhg / 760 mmhg/atm ≈ 0.645 atm. If the total pressure in the tank is 490. The pressure of helium is given as 490 mmhg. 96% (50 ratings) share share share done loading. What is the total pressure in mmhg? Convert the pressure of helium from mmhg to.
from www.numerade.com
A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520 mmhg. Here’s how to approach this question. First, convert the pressure of helium from mmhg to atm: To convert mmhg to atm, use the. The total pressure in the tank, which contains helium at 490 mmhg, nitrogen at 0.75 atm, and neon at 520 torr, is 2.1 atm. 1 atm = 760 mmhg so, helium pressure in atm = 490 mmhg / 760 mmhg/atm ≈ 0.645 atm. The pressure of helium is given as 490 mmhg. If the total pressure in the tank is 490. Convert the pressure of helium from mmhg to atm. Mmhg and the partial pressures of.
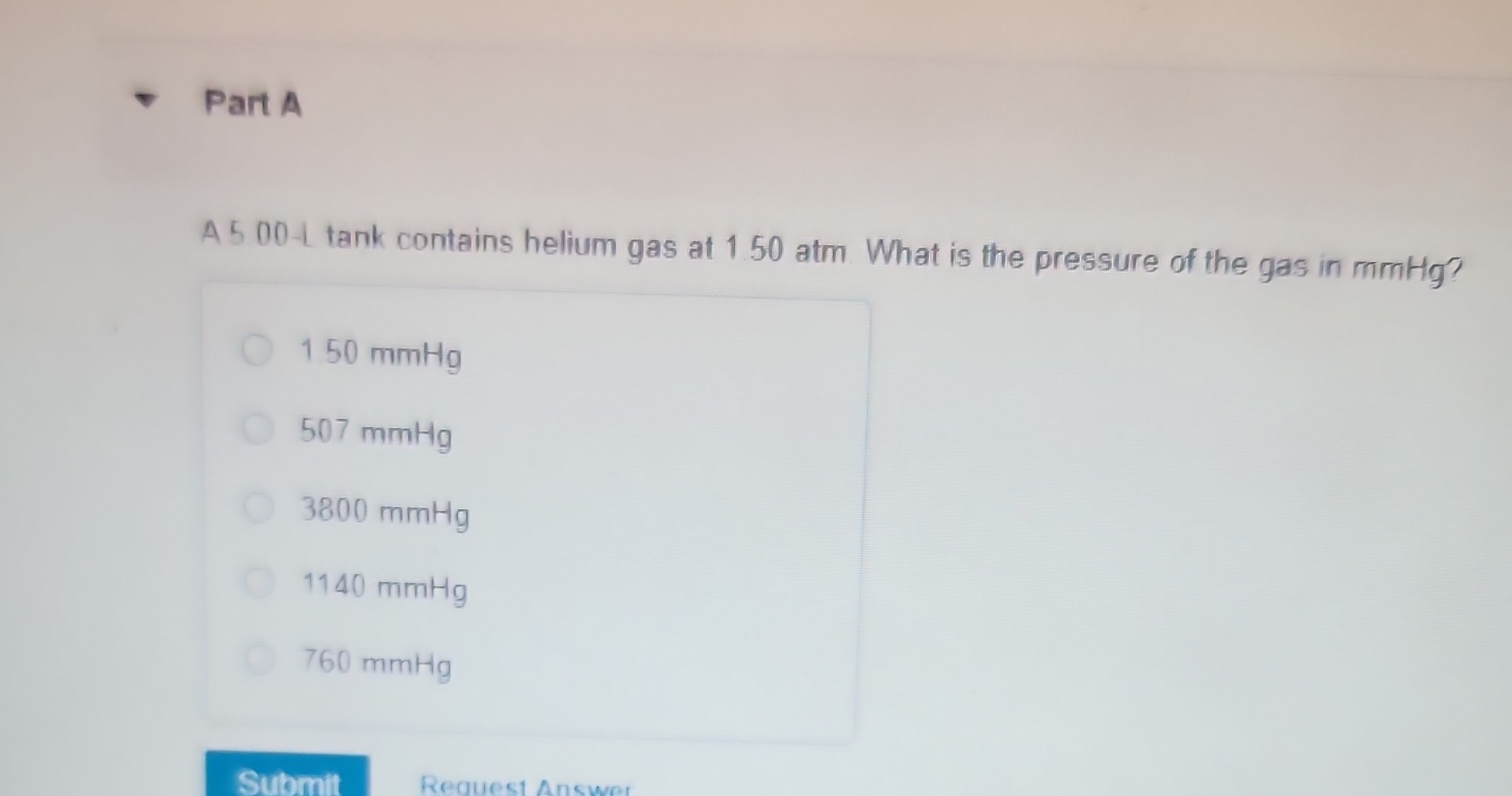
Part A A 500t tank contains helium gas at 150 atm. What is the
A Tank Contains Helium Gas At 490 Mmhg Convert the pressure of helium from mmhg to atm. (1 atm = 760 mmhg) A tank contains a mixture of helium, neon, and argon gases. If the total pressure in the tank is 490. What is the total pressure in mmhg? 96% (50 ratings) share share share done loading. Convert the pressure of helium from mmhg to atm. Convert the pressure of helium from mmhg to. To convert mmhg to atm, use the. First, convert the pressure of helium from mmhg to atm: The pressure of helium is given as 490 mmhg. A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520 mmhg. 1 atm = 760 mmhg so, helium pressure in atm = 490 mmhg / 760 mmhg/atm ≈ 0.645 atm. Here’s how to approach this question. The total pressure in the tank, which contains helium at 490 mmhg, nitrogen at 0.75 atm, and neon at 520 torr, is 2.1 atm. Mmhg and the partial pressures of.
From www.chegg.com
Solved 1. A tank has a volume of 0.1 m and contains helium A Tank Contains Helium Gas At 490 Mmhg 1 atm = 760 mmhg so, helium pressure in atm = 490 mmhg / 760 mmhg/atm ≈ 0.645 atm. The total pressure in the tank, which contains helium at 490 mmhg, nitrogen at 0.75 atm, and neon at 520 torr, is 2.1 atm. A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved 1 of 24 onstants 1 Periodic Table art A A tank A Tank Contains Helium Gas At 490 Mmhg Convert the pressure of helium from mmhg to. Here’s how to approach this question. Mmhg and the partial pressures of. 96% (50 ratings) share share share done loading. First, convert the pressure of helium from mmhg to atm: If the total pressure in the tank is 490. (1 atm = 760 mmhg) What is the total pressure in mmhg? 1. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved A 10.00L tank contains helium gas at 2.5atm. What is A Tank Contains Helium Gas At 490 Mmhg To convert mmhg to atm, use the. A tank contains a mixture of helium, neon, and argon gases. Here’s how to approach this question. Convert the pressure of helium from mmhg to atm. 1 atm = 760 mmhg so, helium pressure in atm = 490 mmhg / 760 mmhg/atm ≈ 0.645 atm. If the total pressure in the tank is. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved 5 of 35 A5.00 L tank contains helium gas at 1.50 atm. A Tank Contains Helium Gas At 490 Mmhg Convert the pressure of helium from mmhg to. The pressure of helium is given as 490 mmhg. (1 atm = 760 mmhg) What is the total pressure in mmhg? A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520 mmhg. Here’s how to approach this question. 96% (50 ratings) share share share done. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved 9) A tank contains helium gas at 490mmHg. nitrogen A Tank Contains Helium Gas At 490 Mmhg What is the total pressure in mmhg? 96% (50 ratings) share share share done loading. First, convert the pressure of helium from mmhg to atm: Mmhg and the partial pressures of. A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520 mmhg. 1 atm = 760 mmhg so, helium pressure in atm =. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved 1 of 24 onstants 1 Periodic Table art A A tank A Tank Contains Helium Gas At 490 Mmhg (1 atm = 760 mmhg) What is the total pressure in mmhg? The total pressure in the tank, which contains helium at 490 mmhg, nitrogen at 0.75 atm, and neon at 520 torr, is 2.1 atm. Mmhg and the partial pressures of. A tank contains a mixture of helium, neon, and argon gases. To convert mmhg to atm, use the.. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved 4. A tank contains helium gas at 490 mm Hg, nitrogen A Tank Contains Helium Gas At 490 Mmhg The pressure of helium is given as 490 mmhg. If the total pressure in the tank is 490. A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520 mmhg. The total pressure in the tank, which contains helium at 490 mmhg, nitrogen at 0.75 atm, and neon at 520 torr, is 2.1 atm.. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved Problem 3 A rigid tank contains helium gas at an A Tank Contains Helium Gas At 490 Mmhg Here’s how to approach this question. Convert the pressure of helium from mmhg to atm. Convert the pressure of helium from mmhg to. The pressure of helium is given as 490 mmhg. 96% (50 ratings) share share share done loading. A tank contains a mixture of helium, neon, and argon gases. Mmhg and the partial pressures of. If the total. A Tank Contains Helium Gas At 490 Mmhg.
From solvedlib.com
A 5.00 L tank of gas contains helium gas at 1.50 atm;… SolvedLib A Tank Contains Helium Gas At 490 Mmhg What is the total pressure in mmhg? 1 atm = 760 mmhg so, helium pressure in atm = 490 mmhg / 760 mmhg/atm ≈ 0.645 atm. (1 atm = 760 mmhg) Convert the pressure of helium from mmhg to. 96% (50 ratings) share share share done loading. A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm,. A Tank Contains Helium Gas At 490 Mmhg.
From www.numerade.com
SOLVED A tank contains helium gas at 490 mmHg; nitrogen gas at 0.75 A Tank Contains Helium Gas At 490 Mmhg To convert mmhg to atm, use the. A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520 mmhg. 96% (50 ratings) share share share done loading. What is the total pressure in mmhg? The pressure of helium is given as 490 mmhg. Convert the pressure of helium from mmhg to. A tank contains. A Tank Contains Helium Gas At 490 Mmhg.
From www.vrogue.co
Solved 1 A Sample Of Argon Gas At Standard Temperatur vrogue.co A Tank Contains Helium Gas At 490 Mmhg Convert the pressure of helium from mmhg to. Here’s how to approach this question. Convert the pressure of helium from mmhg to atm. What is the total pressure in mmhg? (1 atm = 760 mmhg) Mmhg and the partial pressures of. If the total pressure in the tank is 490. To convert mmhg to atm, use the. The total pressure. A Tank Contains Helium Gas At 490 Mmhg.
From www.numerade.com
SOLVED A tank contains helium gas at 490 mmHg, nitrogen gas at 0.75 A Tank Contains Helium Gas At 490 Mmhg Here’s how to approach this question. To convert mmhg to atm, use the. 96% (50 ratings) share share share done loading. The pressure of helium is given as 490 mmhg. A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520 mmhg. 1 atm = 760 mmhg so, helium pressure in atm = 490. A Tank Contains Helium Gas At 490 Mmhg.
From www.numerade.com
SOLVED A steel tank contains a mixture of Ar and He gases. The total A Tank Contains Helium Gas At 490 Mmhg Mmhg and the partial pressures of. Convert the pressure of helium from mmhg to atm. Here’s how to approach this question. A tank contains a mixture of helium, neon, and argon gases. First, convert the pressure of helium from mmhg to atm: If the total pressure in the tank is 490. What is the total pressure in mmhg? The total. A Tank Contains Helium Gas At 490 Mmhg.
From www.numerade.com
SOLVED A tank contains a mixture of helium, neon, and argon gases. If A Tank Contains Helium Gas At 490 Mmhg Here’s how to approach this question. The total pressure in the tank, which contains helium at 490 mmhg, nitrogen at 0.75 atm, and neon at 520 torr, is 2.1 atm. First, convert the pressure of helium from mmhg to atm: Mmhg and the partial pressures of. Convert the pressure of helium from mmhg to. (1 atm = 760 mmhg) To. A Tank Contains Helium Gas At 490 Mmhg.
From www.numerade.com
SOLVED A tank contains a mixture of helium, neon, and argon gases. If A Tank Contains Helium Gas At 490 Mmhg The total pressure in the tank, which contains helium at 490 mmhg, nitrogen at 0.75 atm, and neon at 520 torr, is 2.1 atm. A tank contains a mixture of helium, neon, and argon gases. Here’s how to approach this question. 1 atm = 760 mmhg so, helium pressure in atm = 490 mmhg / 760 mmhg/atm ≈ 0.645 atm.. A Tank Contains Helium Gas At 490 Mmhg.
From www.numerade.com
Application of Boyle's law A 12liter tank contains helium gas A Tank Contains Helium Gas At 490 Mmhg First, convert the pressure of helium from mmhg to atm: Mmhg and the partial pressures of. The total pressure in the tank, which contains helium at 490 mmhg, nitrogen at 0.75 atm, and neon at 520 torr, is 2.1 atm. Here’s how to approach this question. 1 atm = 760 mmhg so, helium pressure in atm = 490 mmhg /. A Tank Contains Helium Gas At 490 Mmhg.
From www.numerade.com
(II) A tank contains 26.0 kg of O2 gas at a gauge pressure of 8.70 atm A Tank Contains Helium Gas At 490 Mmhg To convert mmhg to atm, use the. (1 atm = 760 mmhg) A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520 mmhg. Here’s how to approach this question. Convert the pressure of helium from mmhg to. A tank contains a mixture of helium, neon, and argon gases. What is the total pressure. A Tank Contains Helium Gas At 490 Mmhg.
From www.bartleby.com
Answered A 5.00L tank contains helium gas at… bartleby A Tank Contains Helium Gas At 490 Mmhg (1 atm = 760 mmhg) Here’s how to approach this question. A tank contains a mixture of helium, neon, and argon gases. 96% (50 ratings) share share share done loading. A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520 mmhg. If the total pressure in the tank is 490. The pressure of. A Tank Contains Helium Gas At 490 Mmhg.
From www.numerade.com
VIDEO solution A tank contains helium gas at 576 mmHg, nitrogen gas at A Tank Contains Helium Gas At 490 Mmhg Mmhg and the partial pressures of. The total pressure in the tank, which contains helium at 490 mmhg, nitrogen at 0.75 atm, and neon at 520 torr, is 2.1 atm. A tank contains a mixture of helium, neon, and argon gases. To convert mmhg to atm, use the. If the total pressure in the tank is 490. A tank contains. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved 8. A tank contains helium gas at 390 mmHg, nitrogen A Tank Contains Helium Gas At 490 Mmhg Convert the pressure of helium from mmhg to atm. First, convert the pressure of helium from mmhg to atm: To convert mmhg to atm, use the. Convert the pressure of helium from mmhg to. What is the total pressure in mmhg? 1 atm = 760 mmhg so, helium pressure in atm = 490 mmhg / 760 mmhg/atm ≈ 0.645 atm.. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved A rigid tank contains helium (molar mass, M = 4 A Tank Contains Helium Gas At 490 Mmhg First, convert the pressure of helium from mmhg to atm: Convert the pressure of helium from mmhg to. (1 atm = 760 mmhg) The pressure of helium is given as 490 mmhg. To convert mmhg to atm, use the. If the total pressure in the tank is 490. Mmhg and the partial pressures of. What is the total pressure in. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved A tank contains helium gas at 0.500 atm at 22.5 K. If A Tank Contains Helium Gas At 490 Mmhg A tank contains a mixture of helium, neon, and argon gases. The total pressure in the tank, which contains helium at 490 mmhg, nitrogen at 0.75 atm, and neon at 520 torr, is 2.1 atm. First, convert the pressure of helium from mmhg to atm: A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved A tank contains helium gas at 490 mmhg, nitrogen gas A Tank Contains Helium Gas At 490 Mmhg A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520 mmhg. The pressure of helium is given as 490 mmhg. What is the total pressure in mmhg? First, convert the pressure of helium from mmhg to atm: Mmhg and the partial pressures of. (1 atm = 760 mmhg) Here’s how to approach this. A Tank Contains Helium Gas At 490 Mmhg.
From www.numerade.com
Part A A 500t tank contains helium gas at 150 atm. What is the A Tank Contains Helium Gas At 490 Mmhg Convert the pressure of helium from mmhg to atm. Here’s how to approach this question. Mmhg and the partial pressures of. What is the total pressure in mmhg? A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520 mmhg. (1 atm = 760 mmhg) If the total pressure in the tank is 490.. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved 1 of 24 onstants 1 Periodic Table art A A tank A Tank Contains Helium Gas At 490 Mmhg A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520 mmhg. Convert the pressure of helium from mmhg to atm. A tank contains a mixture of helium, neon, and argon gases. Here’s how to approach this question. 96% (50 ratings) share share share done loading. What is the total pressure in mmhg? First,. A Tank Contains Helium Gas At 490 Mmhg.
From www.coursehero.com
[Solved] A 5.00L tank contains helium gas at 1.50 atm. What is the A Tank Contains Helium Gas At 490 Mmhg The pressure of helium is given as 490 mmhg. First, convert the pressure of helium from mmhg to atm: Convert the pressure of helium from mmhg to atm. Convert the pressure of helium from mmhg to. A tank contains a mixture of helium, neon, and argon gases. (1 atm = 760 mmhg) If the total pressure in the tank is. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved 2. A tank contains helium gas at 1.50 atm. What is A Tank Contains Helium Gas At 490 Mmhg Mmhg and the partial pressures of. 96% (50 ratings) share share share done loading. What is the total pressure in mmhg? 1 atm = 760 mmhg so, helium pressure in atm = 490 mmhg / 760 mmhg/atm ≈ 0.645 atm. Convert the pressure of helium from mmhg to. If the total pressure in the tank is 490. (1 atm =. A Tank Contains Helium Gas At 490 Mmhg.
From www.vrogue.co
2 A 10 0 L Tank Contains Helium Gas At 2 20 Atm What vrogue.co A Tank Contains Helium Gas At 490 Mmhg (1 atm = 760 mmhg) A tank contains a mixture of helium, neon, and argon gases. To convert mmhg to atm, use the. What is the total pressure in mmhg? Convert the pressure of helium from mmhg to. A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520 mmhg. 1 atm = 760. A Tank Contains Helium Gas At 490 Mmhg.
From www.numerade.com
SOLVED 3. A 7.50 liter tank holds helium gas at 1.75 atm. What is the A Tank Contains Helium Gas At 490 Mmhg A tank contains a mixture of helium, neon, and argon gases. What is the total pressure in mmhg? Convert the pressure of helium from mmhg to. (1 atm = 760 mmhg) To convert mmhg to atm, use the. The pressure of helium is given as 490 mmhg. Here’s how to approach this question. Convert the pressure of helium from mmhg. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved A tank contains helium gas at 490 mmHg, nitrogen gas A Tank Contains Helium Gas At 490 Mmhg The pressure of helium is given as 490 mmhg. Here’s how to approach this question. First, convert the pressure of helium from mmhg to atm: What is the total pressure in mmhg? (1 atm = 760 mmhg) Mmhg and the partial pressures of. Convert the pressure of helium from mmhg to atm. To convert mmhg to atm, use the. The. A Tank Contains Helium Gas At 490 Mmhg.
From www.numerade.com
SOLVED A tank having a volume of 0.100 m^3 contains helium gas at 150 A Tank Contains Helium Gas At 490 Mmhg Mmhg and the partial pressures of. The total pressure in the tank, which contains helium at 490 mmhg, nitrogen at 0.75 atm, and neon at 520 torr, is 2.1 atm. Convert the pressure of helium from mmhg to atm. What is the total pressure in mmhg? A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and. A Tank Contains Helium Gas At 490 Mmhg.
From www.numerade.com
SOLVED A 5.00L tank contains helium gas at 0.450 atm. What is the A Tank Contains Helium Gas At 490 Mmhg First, convert the pressure of helium from mmhg to atm: The total pressure in the tank, which contains helium at 490 mmhg, nitrogen at 0.75 atm, and neon at 520 torr, is 2.1 atm. 96% (50 ratings) share share share done loading. What is the total pressure in mmhg? Mmhg and the partial pressures of. A tank contains a mixture. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved 1 of 24 onstants 1 Periodic Table art A A tank A Tank Contains Helium Gas At 490 Mmhg To convert mmhg to atm, use the. 96% (50 ratings) share share share done loading. If the total pressure in the tank is 490. What is the total pressure in mmhg? First, convert the pressure of helium from mmhg to atm: A tank contains helium gas at 490 mmhg, nitrogen gas at 0.75 atm, and neon at 520 mmhg. (1. A Tank Contains Helium Gas At 490 Mmhg.
From www.numerade.com
SOLVED The tank contains a mixture of helium, neon, and argon gas A Tank Contains Helium Gas At 490 Mmhg A tank contains a mixture of helium, neon, and argon gases. The pressure of helium is given as 490 mmhg. 96% (50 ratings) share share share done loading. First, convert the pressure of helium from mmhg to atm: The total pressure in the tank, which contains helium at 490 mmhg, nitrogen at 0.75 atm, and neon at 520 torr, is. A Tank Contains Helium Gas At 490 Mmhg.
From www.chegg.com
Solved D Question 35 2.5 pts A tank contains helium gas at A Tank Contains Helium Gas At 490 Mmhg A tank contains a mixture of helium, neon, and argon gases. Convert the pressure of helium from mmhg to atm. The pressure of helium is given as 490 mmhg. To convert mmhg to atm, use the. 96% (50 ratings) share share share done loading. What is the total pressure in mmhg? First, convert the pressure of helium from mmhg to. A Tank Contains Helium Gas At 490 Mmhg.