What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance . Explain what the term colligative means, and list the colligative properties. You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; At the boiling point, once again,. After all the solid has melted, once again, the heat added goes to increasing the kinetic energy (and temperature) of the liquid molecules until the boiling point. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. The temperature of the liquid water continues to increase as heat is added, until it reaches its boiling point of 100 °c. If you add salt to water, you raise the boiling point, or the temperature at which water boils.
from www.learnatnoon.com
If you add salt to water, you raise the boiling point, or the temperature at which water boils. At the boiling point, once again,. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. After all the solid has melted, once again, the heat added goes to increasing the kinetic energy (and temperature) of the liquid molecules until the boiling point. Explain what the term colligative means, and list the colligative properties. The temperature of the liquid water continues to increase as heat is added, until it reaches its boiling point of 100 °c. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time;
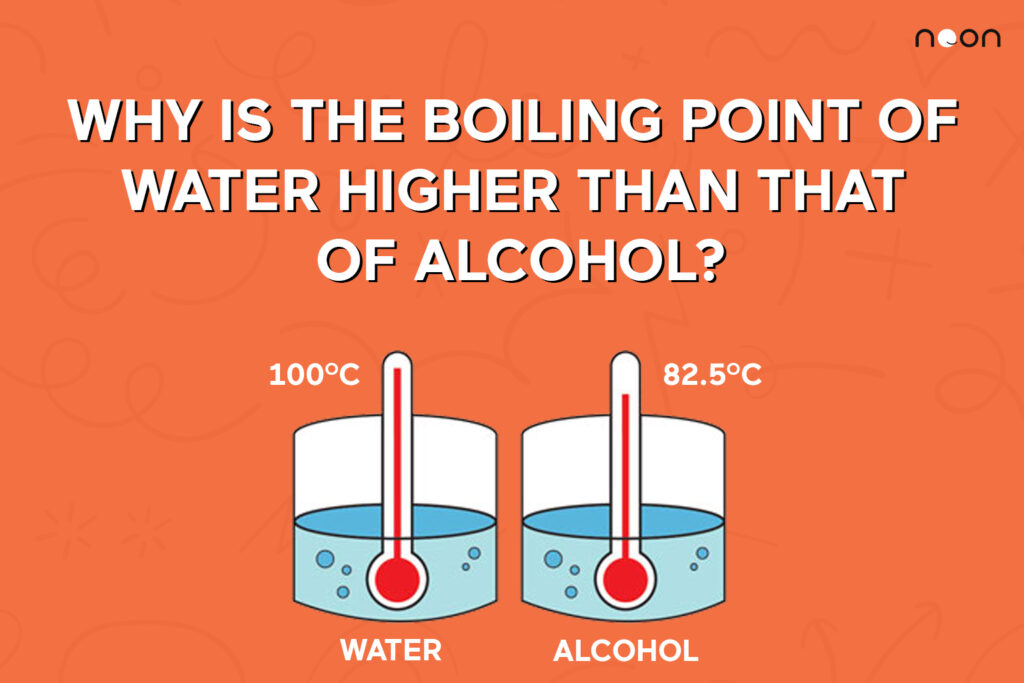
The boiling point of water and alcohol explained Noon Academy
What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance Explain what the term colligative means, and list the colligative properties. You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; Explain what the term colligative means, and list the colligative properties. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. After all the solid has melted, once again, the heat added goes to increasing the kinetic energy (and temperature) of the liquid molecules until the boiling point. At the boiling point, once again,. The temperature of the liquid water continues to increase as heat is added, until it reaches its boiling point of 100 °c. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the.
From ar.inspiredpencil.com
Boiling Point Of Water Examples What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. If you add salt to water, you raise the boiling point, or the temperature at which water boils. At the boiling point, once again,. The temperature needed to boil will increase by about 0.5 c for every 58. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.youtube.com
Does Salt Water Boil Faster? Experiment YouTube What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance At the boiling point, once again,. Explain what the term colligative means, and list the colligative properties. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature needed. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.numerade.com
Attempt 3 Arrange the boiling points of the aqueous solutions relative to pure water. Assume What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance If you add salt to water, you raise the boiling point, or the temperature at which water boils. After all the solid has melted, once again, the heat added goes to increasing the kinetic energy (and temperature) of the liquid molecules until the boiling point. The temperature needed to boil will increase by about 0.5 c for every 58 grams. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From pubchem.ncbi.nlm.nih.gov
Boiling Point Periodic Table of Elements PubChem What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance The temperature needed to boil will increase by about 0.5 c for every 58 grams of. After all the solid has melted, once again, the heat added goes to increasing the kinetic energy (and temperature) of the liquid molecules until the boiling point. You probably learned from unintentional play at the kitchen table as a child that if you sprinkle. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From slideplayer.com
Physical Properties of Matter ppt download What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance Explain what the term colligative means, and list the colligative properties. At the boiling point, once again,. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. The temperature of the liquid water continues to increase as heat is added, until it reaches its boiling point of 100. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free download ID1989506 What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance Explain what the term colligative means, and list the colligative properties. At the boiling point, once again,. After all the solid has melted, once again, the heat added goes to increasing the kinetic energy (and temperature) of the liquid molecules until the boiling point. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. The temperature of the liquid water continues to increase as heat is added, until it reaches its boiling point of 100 °c. At the boiling point, once again,. You probably learned from unintentional play at the kitchen table. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance At the boiling point, once again,. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. If you add salt to water, you raise the boiling point, or the temperature at which water boils. You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.slideserve.com
PPT MATTER AND TEMPERATURE PowerPoint Presentation, free download ID6401941 What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance Explain what the term colligative means, and list the colligative properties. After all the solid has melted, once again, the heat added goes to increasing the kinetic energy (and temperature) of the liquid molecules until the boiling point. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Pure water has a boiling point. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance Explain what the term colligative means, and list the colligative properties. At the boiling point, once again,. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance After all the solid has melted, once again, the heat added goes to increasing the kinetic energy (and temperature) of the liquid molecules until the boiling point. If you add salt to water, you raise the boiling point, or the temperature at which water boils. At the boiling point, once again,. Pure water has a boiling point of 100°c at. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From pakmcqs.com
Boiling point of pure water is __________? PakMcqs What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; After all the solid has melted, once again,. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From askfilo.com
The normal boiling point of pure water is 373.15 K. Calculate the boiling.. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance Explain what the term colligative means, and list the colligative properties. After all the solid has melted, once again, the heat added goes to increasing the kinetic energy (and temperature) of the liquid molecules until the boiling point. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. If you add salt to water,. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From brainly.com
Arrange the boiling points of the aqueous solutions, relative to pure water. Assume complete What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance At the boiling point, once again,. After all the solid has melted, once again, the heat added goes to increasing the kinetic energy (and temperature) of the liquid molecules until the boiling point. You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.slideserve.com
PPT Opening Assignment PowerPoint Presentation, free download ID3170025 What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. After all the solid has melted, once again,. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance The temperature needed to boil will increase by about 0.5 c for every 58 grams of. You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; The temperature of the liquid water continues to increase as heat is added, until. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.chegg.com
Solved Arrange the boiling points of the aqueous solutions, What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. At the boiling point, once again,. The temperature of the liquid water continues to increase as heat is added, until it reaches its boiling point of 100 °c. If you add salt to water, you raise the boiling. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.slideshare.net
Purification Of Substances 1 What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; The temperature needed to boil will increase by about 0.5 c for every 58 grams of. At the boiling point, once again,. After all the solid has melted, once again,. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting boiling point of liquids What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; The temperature of the liquid water continues to increase as heat is added, until it reaches its boiling point of 100 °c. Pure water has a boiling point of 100°c. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.animalia-life.club
Boiling Point Of Water Examples What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; At the boiling point, once again,. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature needed to boil will. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; The temperature of the liquid water continues to increase as heat is added, until it reaches its boiling point of 100 °c. After all the solid has melted, once again,. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.animalia-life.club
Boiling Point Of Water For Kids What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance The temperature needed to boil will increase by about 0.5 c for every 58 grams of. The temperature of the liquid water continues to increase as heat is added, until it reaches its boiling point of 100 °c. You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.slideserve.com
PPT Opening Assignment PowerPoint Presentation, free download ID3170025 What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance If you add salt to water, you raise the boiling point, or the temperature at which water boils. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. At the boiling point, once again,. Explain what the term colligative means, and list the colligative properties. After all the. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Explain what the term colligative means, and list the colligative properties. The temperature needed to boil will increase by about. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free download ID2755993 What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; Explain what the term colligative means, and list the colligative properties. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Pure. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock Vector Adobe Stock What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. The temperature of the liquid water continues to increase as heat is added, until it reaches its boiling point of 100 °c. The temperature needed to boil will increase by about 0.5 c for every 58 grams of.. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From lyndajguilleno.blob.core.windows.net
What Happens To Water When It Reaches Its Boiling Point at lyndajguilleno blog What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance After all the solid has melted, once again, the heat added goes to increasing the kinetic energy (and temperature) of the liquid molecules until the boiling point. At the boiling point, once again,. You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.worldatlas.com
What is the Boiling Point of Water? WorldAtlas What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance If you add salt to water, you raise the boiling point, or the temperature at which water boils. You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; The temperature of the liquid water continues to increase as heat is. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.slideserve.com
PPT Physical Properties of Water Boiling Point, Melting Point and Freezing Point PowerPoint What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance At the boiling point, once again,. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature of the liquid water continues to increase as heat is added, until it reaches its boiling point of 100 °c. After all the solid has melted, once again, the heat added goes to increasing. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From mavink.com
Elevation In Boiling Point Graph What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance The temperature of the liquid water continues to increase as heat is added, until it reaches its boiling point of 100 °c. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. Explain what the term colligative means, and list the colligative properties. The temperature needed to boil. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.slideserve.com
PPT Chapter 13 continued… PowerPoint Presentation, free download ID1552081 What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. At the boiling point, once again,. Explain what. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.tes.com
Lesson Pure and Impure GSCE Edexcel 91 by chemistryteacher001 Teaching Resources Tes What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance After all the solid has melted, once again, the heat added goes to increasing the kinetic energy (and temperature) of the liquid molecules until the boiling point. The temperature of the liquid water continues to increase as heat is added, until it reaches its boiling point of 100 °c. At the boiling point, once again,. If you add salt to. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.youtube.com
COMPARING BOILING POINTS OF PURE SUBSTANCE YouTube What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. After all the solid has melted, once again, the heat added goes to increasing the kinetic energy (and temperature) of the liquid molecules until the boiling point. At the boiling point, once again,. The temperature of the liquid. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.worksheetsplanet.com
What is Boiling Point What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance At the boiling point, once again,. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.
From www.thoughtco.com
What Is the Boiling Point of Water? What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; Explain what the term colligative means, and list the colligative properties. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. At the boiling. What Happened To The Boiling Point Of The Pure Water When You Added A Solid Substance.