Lactic Acid Naoh Titration Equivalence Point . The concentration of sodium lactate is given by the equation: In other words, while titrating, it is a point. Suppose that a titration is performed and \(20.70 \: Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1 n naoh corresponds to 0.009008 g of lactic acid. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Naoh and h 2 so 4 react in a 2:1 molar ratio. \ce{naoh}\) is required to reach the end point when titrated.
from general.chemistrysteps.com
Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. In other words, while titrating, it is a point. Naoh and h 2 so 4 react in a 2:1 molar ratio. The concentration of sodium lactate is given by the equation: The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1 n naoh corresponds to 0.009008 g of lactic acid. Suppose that a titration is performed and \(20.70 \: To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. \ce{naoh}\) is required to reach the end point when titrated.
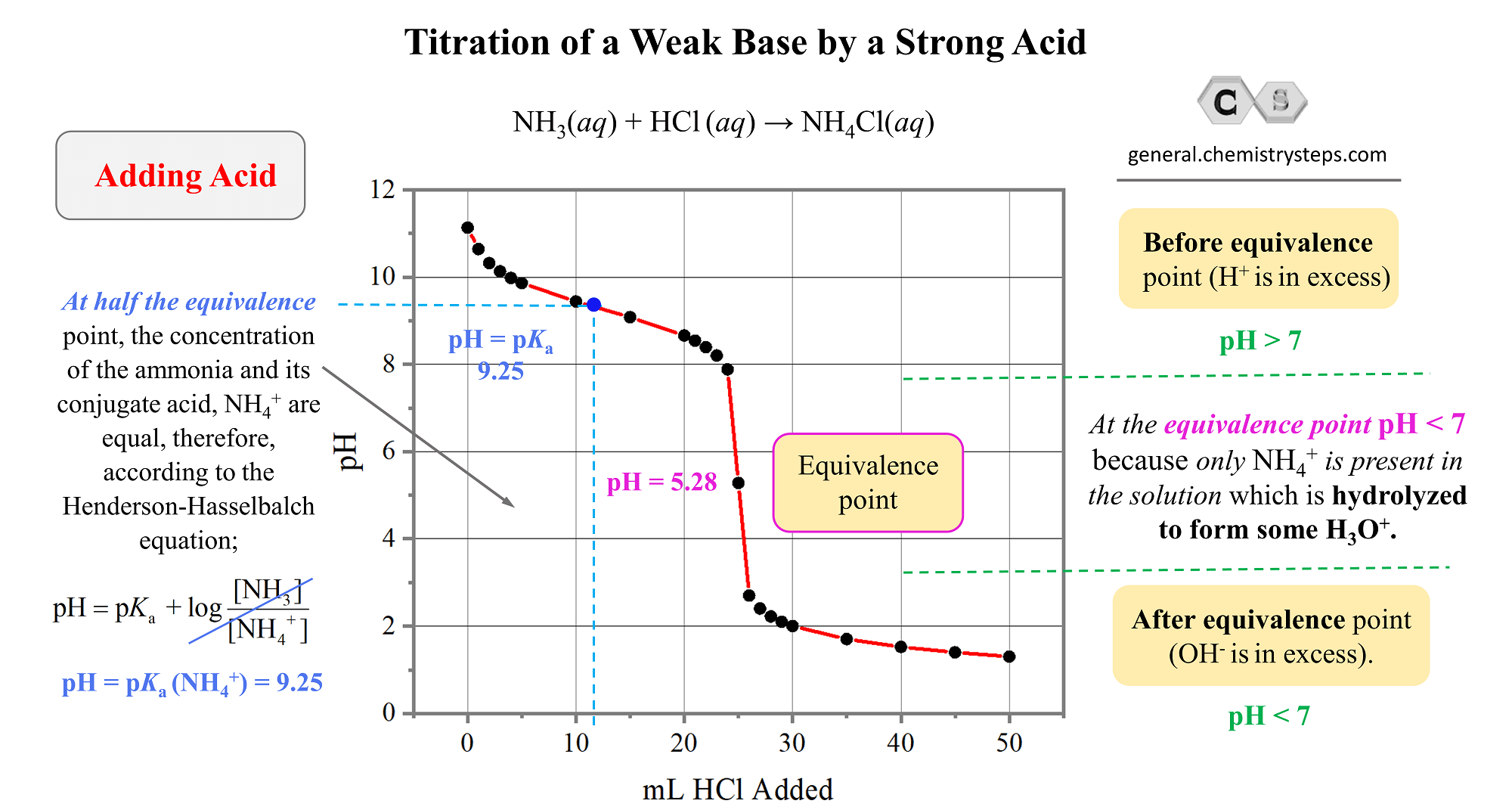
Titration of a Weak Base by a Strong Acid Chemistry Steps
Lactic Acid Naoh Titration Equivalence Point In other words, while titrating, it is a point. In other words, while titrating, it is a point. The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1 n naoh corresponds to 0.009008 g of lactic acid. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The concentration of sodium lactate is given by the equation: \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \: Naoh and h 2 so 4 react in a 2:1 molar ratio.
From www.numerade.com
SOLVED Q2) The titratable acidity is expressed as 2/100 mL of lactic Lactic Acid Naoh Titration Equivalence Point Suppose that a titration is performed and \(20.70 \: Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it is a point. The concentration of. Lactic Acid Naoh Titration Equivalence Point.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Lactic Acid Naoh Titration Equivalence Point In other words, while titrating, it is a point. Naoh and h 2 so 4 react in a 2:1 molar ratio. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Calculate. Lactic Acid Naoh Titration Equivalence Point.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass Lactic Acid Naoh Titration Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. In other words, while titrating, it is a point. Naoh and h 2 so 4 react in a 2:1 molar ratio.. Lactic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED Question 1 1 pts What are the correct definitions of Lactic Acid Naoh Titration Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The concentration of sodium lactate is given by the equation: The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1 n naoh corresponds to 0.009008 g of lactic acid. To achieve neutralization, 0.00700 mol of naoh reacts with. Lactic Acid Naoh Titration Equivalence Point.
From www.scribd.com
AcidBase Titration Using PH Meter and Finding The Equivalence Point Lactic Acid Naoh Titration Equivalence Point Suppose that a titration is performed and \(20.70 \: The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1 n naoh corresponds to 0.009008 g of lactic acid. In other words, while titrating, it is a point. To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. Naoh and h 2. Lactic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED Explain why your diluted solution of NaOH needs to be Lactic Acid Naoh Titration Equivalence Point \ce{naoh}\) is required to reach the end point when titrated. The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1 n naoh corresponds to 0.009008 g of lactic acid. In other words, while titrating, it is a point. To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. The concentration of. Lactic Acid Naoh Titration Equivalence Point.
From www.numerade.com
VIDEO solution A series of titrations of lactic acid, CH3CH(OH) COOH Lactic Acid Naoh Titration Equivalence Point Naoh and h 2 so 4 react in a 2:1 molar ratio. To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. \ce{naoh}\) is required to reach the end point when titrated. The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1 n naoh corresponds to 0.009008 g of lactic acid.. Lactic Acid Naoh Titration Equivalence Point.
From schoolbag.info
Titration and Buffers Acids and Bases Lactic Acid Naoh Titration Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Naoh and h 2 so 4 react in a 2:1 molar ratio. Suppose that a titration is performed and \(20.70 \: To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. Calculate ph at the. Lactic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED Consider the titration curve below for the titration of oxalic Lactic Acid Naoh Titration Equivalence Point \ce{naoh}\) is required to reach the end point when titrated. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The key stoichiometric equivalence for this titration is that 1.0 ml. Lactic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVEDA series of titrations of lactic acid, CH3 CH(OH) COOH (p Ka=3. Lactic Acid Naoh Titration Equivalence Point In other words, while titrating, it is a point. The concentration of sodium lactate is given by the equation: Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. The key stoichiometric equivalence for. Lactic Acid Naoh Titration Equivalence Point.
From www.researchgate.net
pH titration curves of free lactic acid and Cr 3þ /lactic acid in 1 Lactic Acid Naoh Titration Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The concentration of sodium lactate is given by the equation: To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant. Lactic Acid Naoh Titration Equivalence Point.
From www.numerade.com
Based on the titration curve shown below, what's the pKa of the acid Lactic Acid Naoh Titration Equivalence Point Suppose that a titration is performed and \(20.70 \: The concentration of sodium lactate is given by the equation: Naoh and h 2 so 4 react in a 2:1 molar ratio. \ce{naoh}\) is required to reach the end point when titrated. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically.. Lactic Acid Naoh Titration Equivalence Point.
From socratic.org
What is the Ka value of this citric acid + NaOH titration? Socratic Lactic Acid Naoh Titration Equivalence Point To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Naoh and h 2 so 4 react in a 2:1 molar ratio. Suppose that a titration is performed and \(20.70 \: In other words, while. Lactic Acid Naoh Titration Equivalence Point.
From www.writework.com
Titration of amino acids WriteWork Lactic Acid Naoh Titration Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. In other words, while titrating, it is a point. The key stoichiometric equivalence for this titration is that 1.0 ml of. Lactic Acid Naoh Titration Equivalence Point.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Lactic Acid Naoh Titration Equivalence Point Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The concentration of sodium lactate is given by the equation: Suppose that a titration is performed and \(20.70 \: The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other. Lactic Acid Naoh Titration Equivalence Point.
From psu.pb.unizin.org
AcidBase Titrations (14.7) Chemistry 110 Lactic Acid Naoh Titration Equivalence Point Naoh and h 2 so 4 react in a 2:1 molar ratio. In other words, while titrating, it is a point. To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \: The equivalence point of. Lactic Acid Naoh Titration Equivalence Point.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Lactic Acid Naoh Titration Equivalence Point Naoh and h 2 so 4 react in a 2:1 molar ratio. \ce{naoh}\) is required to reach the end point when titrated. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Suppose that a titration is performed and \(20.70 \: Calculate ph at the equivalence point of formic acid titration. Lactic Acid Naoh Titration Equivalence Point.
From mavink.com
Acid Base Titration Curve Lactic Acid Naoh Titration Equivalence Point The concentration of sodium lactate is given by the equation: The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Suppose that a titration is performed and \(20.70 \: Naoh and h 2 so 4 react in a 2:1 molar ratio. \ce{naoh}\) is required to reach the end point when titrated.. Lactic Acid Naoh Titration Equivalence Point.
From www.vrogue.co
Point In Acid Base Titration The Equivalence Point So vrogue.co Lactic Acid Naoh Titration Equivalence Point To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1 n naoh corresponds to 0.009008 g of lactic acid. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. \ce{naoh}\) is. Lactic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED Lactic acid is a monoprotic acid produced in muscle tissue Lactic Acid Naoh Titration Equivalence Point \ce{naoh}\) is required to reach the end point when titrated. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The concentration of sodium lactate is given by the equation: In other words, while titrating, it is a point. Suppose that a titration is performed and \(20.70 \: To achieve. Lactic Acid Naoh Titration Equivalence Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Lactic Acid Naoh Titration Equivalence Point To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. \ce{naoh}\) is required to reach the end point when titrated. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1 n naoh. Lactic Acid Naoh Titration Equivalence Point.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Lactic Acid Naoh Titration Equivalence Point Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. The concentration of sodium lactate is given by the equation: Naoh and h 2 so 4 react in a 2:1 molar ratio. In other words, while titrating, it is a point. Calculate ph at the equivalence point of formic acid titration. Lactic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVEDTitration Problem Lactic acid, HC3H5O3 (see right for structure Lactic Acid Naoh Titration Equivalence Point Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. Suppose that a titration is performed and \(20.70 \: The concentration of sodium lactate is given by the equation: Naoh and h 2 so 4 react in a 2:1 molar ratio. The key stoichiometric equivalence for this titration is that. Lactic Acid Naoh Titration Equivalence Point.
From www.studeersnel.nl
Acid Base Lab lactic acid titration lab report Acid Base Titrations Lactic Acid Naoh Titration Equivalence Point Naoh and h 2 so 4 react in a 2:1 molar ratio. The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1 n naoh corresponds to 0.009008 g of lactic acid. In other words, while titrating, it is a point. To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. Suppose. Lactic Acid Naoh Titration Equivalence Point.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Lactic Acid Naoh Titration Equivalence Point Suppose that a titration is performed and \(20.70 \: In other words, while titrating, it is a point. To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. The concentration of sodium lactate is given by the equation: The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1 n naoh corresponds. Lactic Acid Naoh Titration Equivalence Point.
From www.researchgate.net
pH titration curve of the aqueous lactic acid solution. The original Lactic Acid Naoh Titration Equivalence Point Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. \ce{naoh}\) is required to reach the end point when titrated. The concentration of sodium lactate is given by the equation: Suppose. Lactic Acid Naoh Titration Equivalence Point.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Lactic Acid Naoh Titration Equivalence Point \ce{naoh}\) is required to reach the end point when titrated. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it is a point. The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1 n naoh corresponds to 0.009008 g of lactic acid.. Lactic Acid Naoh Titration Equivalence Point.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts Lactic Acid Naoh Titration Equivalence Point The concentration of sodium lactate is given by the equation: Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1 n naoh corresponds to 0.009008 g of lactic acid. In other words, while titrating, it is a point.. Lactic Acid Naoh Titration Equivalence Point.
From goodttorials.blogspot.com
How To Find Equivalence Point From Titration Data Lactic Acid Naoh Titration Equivalence Point Naoh and h 2 so 4 react in a 2:1 molar ratio. To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. \ce{naoh}\) is required to reach the end point when titrated. In other words, while titrating, it is a point. The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1. Lactic Acid Naoh Titration Equivalence Point.
From slideplayer.com
Titrations pH Titrant volume, mL ppt download Lactic Acid Naoh Titration Equivalence Point The concentration of sodium lactate is given by the equation: In other words, while titrating, it is a point. Suppose that a titration is performed and \(20.70 \: Naoh and h 2 so 4 react in a 2:1 molar ratio. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. \ce{naoh}\). Lactic Acid Naoh Titration Equivalence Point.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Lactic Acid Naoh Titration Equivalence Point To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The concentration of sodium lactate is given by the equation: \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is. Lactic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED A 40 mL solution of .750 M acetic acid (Ka=1.76x10^5) is Lactic Acid Naoh Titration Equivalence Point In other words, while titrating, it is a point. To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. The concentration of sodium lactate is given by the equation: Suppose that a titration is performed and \(20.70 \: The equivalence point of a chemical reaction is the point at which equal quantities of reactants. Lactic Acid Naoh Titration Equivalence Point.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Lactic Acid Naoh Titration Equivalence Point Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. The concentration of sodium lactate is given by the equation: The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1 n naoh corresponds to 0.009008 g of lactic acid. Naoh and h 2 so 4 react in a. Lactic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED The pH curves for the titrations of various monoprotic acids (A Lactic Acid Naoh Titration Equivalence Point To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. Suppose that a titration is performed and \(20.70 \: In other words, while titrating, it is a point. The concentration of sodium lactate is given by the equation: The key stoichiometric equivalence for this titration is that 1.0 ml of 0.1 n naoh corresponds. Lactic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED When examining the titrations of hydrochloric acid and acetic Lactic Acid Naoh Titration Equivalence Point Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. \ce{naoh}\) is required to reach the end point when titrated. The concentration of sodium lactate is given by the equation: To achieve neutralization, 0.00700 mol of naoh reacts with 0.00350 mole of h 2 so 4. The equivalence point of. Lactic Acid Naoh Titration Equivalence Point.