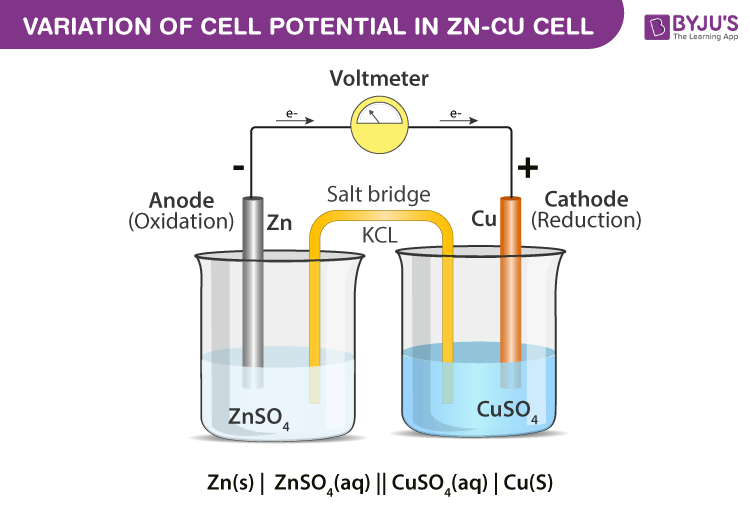
Zinc And Copper Equation . In this equation, zinc (zn) is oxidized while copper. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. The zinc and copper redox equation is written as follows: The solution gradually acquires the blue. 2zn + cu2+ → 2zn2+ + cu. Zinc and copper redox reaction equation. Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution.
from byjus.com
Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. In this equation, zinc (zn) is oxidized while copper. The zinc and copper redox equation is written as follows: Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. Zinc and copper redox reaction equation. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The solution gradually acquires the blue. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +.
Daniell Cell Definition, Construction & Working with Cell Reactions
Zinc And Copper Equation Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Find out what type of reaction occured. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Balance any equation or reaction using this chemical equation balancer! 2zn + cu2+ → 2zn2+ + cu. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. The zinc and copper redox equation is written as follows: The solution gradually acquires the blue. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. Zinc and copper redox reaction equation. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. In this equation, zinc (zn) is oxidized while copper.
From slideplayer.com
Look over objectives and try those types of ppt download Zinc And Copper Equation In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. In this equation, zinc (zn) is oxidized while copper. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous. Zinc And Copper Equation.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc And Copper Equation Zinc and copper redox reaction equation. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The zinc and copper redox equation is written as follows: There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Balance any equation or reaction using this. Zinc And Copper Equation.
From www.coursehero.com
[Solved] For the reaction between zinc and copper(II) sulfate, describe Zinc And Copper Equation There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. In this equation, zinc (zn) is oxidized while copper. The zinc and copper redox equation is written as follows: Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn]. Zinc And Copper Equation.
From www.youtube.com
Determination of Standard Electrode potential of Zinc and Copper YouTube Zinc And Copper Equation The zinc and copper redox equation is written as follows: Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. 2zn + cu2+ → 2zn2+ + cu. Zinc and copper redox reaction equation. In the zn/cu system, the valence electrons in zinc have a substantially higher. Zinc And Copper Equation.
From www.youtube.com
How to Balance Zn + CuCl2 = ZnCl2 + Cu Zinc + Copper (II) chloride Zinc And Copper Equation In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. 2zn + cu2+ → 2zn2+ + cu. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Zinc and copper redox reaction equation. In this equation, zinc (zn) is oxidized. Zinc And Copper Equation.
From www.pastpapersinside.com
Redox Reaction Past Papers Inside Zinc And Copper Equation The zinc and copper redox equation is written as follows: A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Find out what type of reaction occured. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. Zinc (zn) is more reactive than copper. Zinc And Copper Equation.
From www.coursehero.com
[Solved] link for the experiment https//youtu.be/ILJhI43wVA Part Zinc And Copper Equation In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. The zinc and copper redox equation is written as follows: Zinc and copper redox reaction equation. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of.. Zinc And Copper Equation.
From www.numerade.com
Write balanced chemical equations for the following word equations from Zinc And Copper Equation Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. In this equation, zinc (zn) is oxidized while copper. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. The solution gradually acquires the blue. The. Zinc And Copper Equation.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Zinc And Copper Equation A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Zinc and copper redox reaction equation. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. Find out what type of reaction occured. There are three main steps for writing the net ionic equation. Zinc And Copper Equation.
From www.gauthmath.com
The equation 0.95c+0.05n=8.87 represents the density of a copperzinc Zinc And Copper Equation The zinc and copper redox equation is written as follows: Balance any equation or reaction using this chemical equation balancer! There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Find out what. Zinc And Copper Equation.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2810901 Zinc And Copper Equation Zinc and copper redox reaction equation. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. In this equation, zinc (zn) is oxidized while copper. The solution gradually acquires the blue. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles. Zinc And Copper Equation.
From www.slideshare.net
Redox= quiz part 1 with answers Zinc And Copper Equation 2zn + cu2+ → 2zn2+ + cu. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The solution gradually acquires the blue. In this equation, zinc (zn) is oxidized while copper. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two. Zinc And Copper Equation.
From www.slideserve.com
PPT Cells and Voltage PowerPoint Presentation ID5231819 Zinc And Copper Equation Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. Zinc and copper redox reaction equation. A simple redox reaction occurs when copper metal is immersed in a solution of. Zinc And Copper Equation.
From www.youtube.com
How to Balance Zn + CuSO4 = Cu + ZnSO4 (Zinc plus Copper (II) Sulfate Zinc And Copper Equation A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The zinc and copper redox equation is written as follows: Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. In this equation, zinc (zn) is oxidized while copper. Zinc. Zinc And Copper Equation.
From hxejkrhaj.blob.core.windows.net
Copper 2 Sulfate Zinc Equation at Viola Smith blog Zinc And Copper Equation Zinc and copper redox reaction equation. In this equation, zinc (zn) is oxidized while copper. 2zn + cu2+ → 2zn2+ + cu. The zinc and copper redox equation is written as follows: In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. Zn + cuso4 = cu2 + znso4 is. Zinc And Copper Equation.
From www.numerade.com
SOLVED The reaction between zinc and copper sulphate is illustrated Zinc And Copper Equation In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. 2zn + cu2+ → 2zn2+ + cu. Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper. Zinc And Copper Equation.
From byjus.com
A piece of brass (alloy of zinc and copper) weighs 12.9 gm in air. When Zinc And Copper Equation Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. Find out what type of reaction occured. In this equation, zinc (zn) is oxidized while copper. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc. Zinc And Copper Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + Cu(NO3)2 = Cu + Zn(NO3)2 Zinc And Copper Equation Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Find out what type of reaction occured. The solution gradually acquires the blue. Zn + cuso4 = cu2 + znso4 is a single displacement. Zinc And Copper Equation.
From www.meritnation.com
write a word equation for the reaction of Zinc with copper sulphate Zinc And Copper Equation There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. Balance any equation or reaction using this chemical equation balancer! Zinc and copper redox reaction equation. Zinc (zn) is. Zinc And Copper Equation.
From www.numerade.com
SOLVED Write a balanced equation for the singlereplacement oxidation Zinc And Copper Equation A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Zinc and copper redox reaction equation. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid. Zinc And Copper Equation.
From www.chegg.com
Solved 761. Zinc metal reacts with aqueous copper(II) Zinc And Copper Equation Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. Balance any equation or reaction using this chemical equation balancer! There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. Zinc and copper redox reaction. Zinc And Copper Equation.
From www.numerade.com
SOLVED Write the Lewis Dot Structure of Copper Sulfate and Zinc Zinc And Copper Equation Find out what type of reaction occured. 2zn + cu2+ → 2zn2+ + cu. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. Balance any. Zinc And Copper Equation.
From byjus.com
Daniell Cell Definition, Construction & Working with Cell Reactions Zinc And Copper Equation The solution gradually acquires the blue. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. Find out what type of reaction occured. In this equation, zinc (zn) is oxidized while copper. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid. Zinc And Copper Equation.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Zinc And Copper Equation Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The solution gradually acquires the blue. Zinc and copper redox reaction equation. Zinc (zn) is more reactive than copper (cu),. Zinc And Copper Equation.
From express.adobe.com
Zinc and Copper Chloride Zinc And Copper Equation In this equation, zinc (zn) is oxidized while copper. Find out what type of reaction occured. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. 2zn + cu2+ → 2zn2+ + cu. Balance any equation or reaction using this chemical equation balancer! In the zn/cu system, the valence. Zinc And Copper Equation.
From www.chegg.com
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II) Zinc And Copper Equation A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. 2zn + cu2+ →. Zinc And Copper Equation.
From blog.thepipingmart.com
Difference Between Copper and Zinc Zinc And Copper Equation Zinc and copper redox reaction equation. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. 2zn + cu2+ → 2zn2+ + cu. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. The zinc and copper redox equation is. Zinc And Copper Equation.
From slideplayer.com
Chemical Reactions & Equations ppt download Zinc And Copper Equation Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. In this equation, zinc (zn) is oxidized while copper. There are three main steps for writing the net ionic equation for zn +. Zinc And Copper Equation.
From www.chegg.com
Solved What is the net ionic equation for the reaction of Zinc And Copper Equation The solution gradually acquires the blue. Balance any equation or reaction using this chemical equation balancer! Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Zinc and copper redox. Zinc And Copper Equation.
From www.youtube.com
What happens when a zinc strip is dipped into a copper sulphate Zinc And Copper Equation Zinc and copper redox reaction equation. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. The zinc and copper redox equation is written as follows: There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. 2zn + cu2+. Zinc And Copper Equation.
From brainly.com
The provided balanced equation applies to the reaction that takes place Zinc And Copper Equation Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. The zinc and copper redox equation is written as follows: 2zn + cu2+ → 2zn2+ + cu. A simple. Zinc And Copper Equation.
From www.slideshare.net
Replacement reactions Zinc And Copper Equation In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. Zinc (zn) is more reactive than copper (cu), therefore it can displace cu from aqueous copper sulfate (cuso 4) solution. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc. Zinc And Copper Equation.
From blog.thepipingmart.com
Classifications of Metals Copper vs. Zinc Zinc And Copper Equation In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. Balance any equation or reaction using this chemical equation balancer! A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. There are three main steps for writing the net ionic equation for zn +. Zinc And Copper Equation.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Copper Zinc And Copper Equation The solution gradually acquires the blue. Zinc and copper redox reaction equation. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. Find out what type of reaction occured. 2zn + cu2+ → 2zn2+ + cu. Zinc (zn) is more reactive than copper (cu), therefore it. Zinc And Copper Equation.
From www.slideshare.net
Redox= quiz part 1 with answers Zinc And Copper Equation The solution gradually acquires the blue. In this equation, zinc (zn) is oxidized while copper. Balance any equation or reaction using this chemical equation balancer! 2zn + cu2+ → 2zn2+ + cu. There are three main steps for writing the net ionic equation for zn + cuso4 = znso4 + cu (zinc +. A simple redox reaction occurs when copper. Zinc And Copper Equation.