What Is The Relationship Between A Base And A Basic Solution . A solution with a high number of hydrogen. when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. Buffers, ph, acids, and bases. The ph of a solution is a measure of the concentration of hydrogen ions in the solution. If you're behind a web filter, please make sure that the domains. if you're seeing this message, it means we're having trouble loading external resources on our website. The poh is convenient to use when finding the hydroxide ion concentration. there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. Because the base soaks up hydrogen ions, the result is.
from studylib.net
Because the base soaks up hydrogen ions, the result is. The ph of a solution is a measure of the concentration of hydrogen ions in the solution. when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. If you're behind a web filter, please make sure that the domains. The poh is convenient to use when finding the hydroxide ion concentration. Buffers, ph, acids, and bases. there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. A solution with a high number of hydrogen. a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. if you're seeing this message, it means we're having trouble loading external resources on our website.
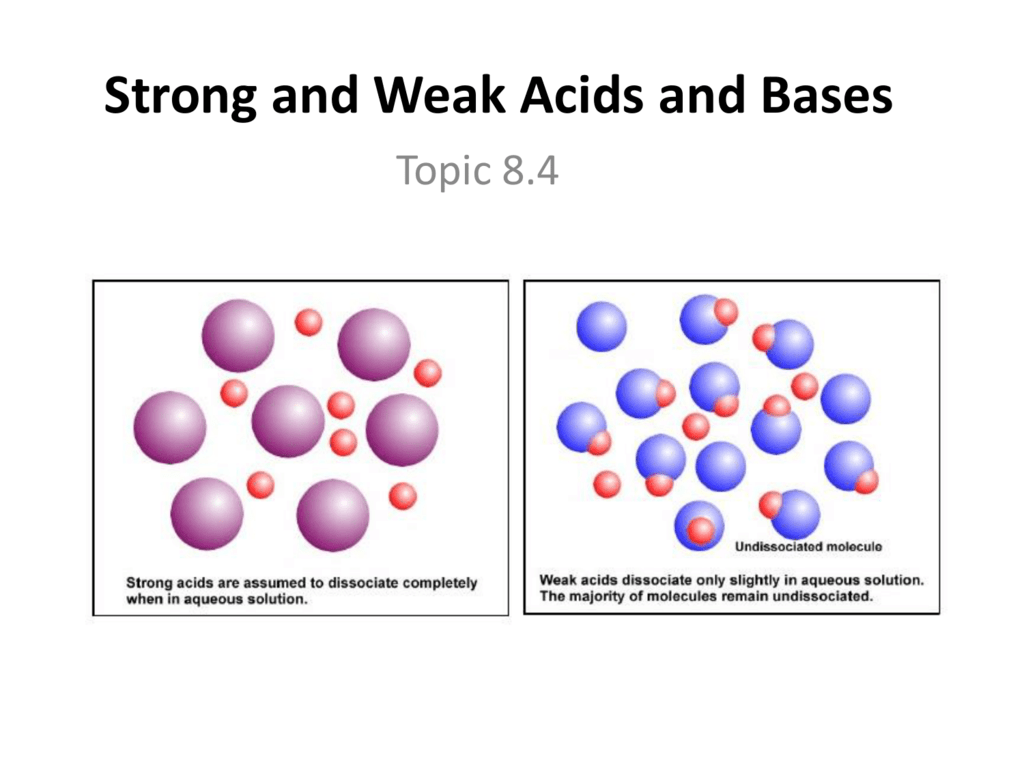
Strong and weak acids and bases
What Is The Relationship Between A Base And A Basic Solution when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. A solution with a high number of hydrogen. If you're behind a web filter, please make sure that the domains. when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. Buffers, ph, acids, and bases. if you're seeing this message, it means we're having trouble loading external resources on our website. Because the base soaks up hydrogen ions, the result is. The ph of a solution is a measure of the concentration of hydrogen ions in the solution. The poh is convenient to use when finding the hydroxide ion concentration. a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs What Is The Relationship Between A Base And A Basic Solution if you're seeing this message, it means we're having trouble loading external resources on our website. A solution with a high number of hydrogen. If you're behind a web filter, please make sure that the domains. Because the base soaks up hydrogen ions, the result is. The ph of a solution is a measure of the concentration of hydrogen. What Is The Relationship Between A Base And A Basic Solution.
From www.slideserve.com
PPT Salts in Solution PowerPoint Presentation, free download ID1820885 What Is The Relationship Between A Base And A Basic Solution A solution with a high number of hydrogen. If you're behind a web filter, please make sure that the domains. a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. Because the base soaks up hydrogen ions, the result is. The ph of a solution is a measure. What Is The Relationship Between A Base And A Basic Solution.
From www.youtube.com
GCSE CHEMISTRY ACIDS AND BASES LESSON 19 salts solubility YouTube What Is The Relationship Between A Base And A Basic Solution if you're seeing this message, it means we're having trouble loading external resources on our website. The poh is convenient to use when finding the hydroxide ion concentration. The ph of a solution is a measure of the concentration of hydrogen ions in the solution. If you're behind a web filter, please make sure that the domains. a. What Is The Relationship Between A Base And A Basic Solution.
From www.reliableeducationgroups.in
Acids, Bases and Salts Class 7 Science Notes Chapter 5 Reliable What Is The Relationship Between A Base And A Basic Solution The ph of a solution is a measure of the concentration of hydrogen ions in the solution. If you're behind a web filter, please make sure that the domains. a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. Buffers, ph, acids, and bases. The poh is convenient. What Is The Relationship Between A Base And A Basic Solution.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry What Is The Relationship Between A Base And A Basic Solution when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. Because the base soaks up hydrogen ions, the result is. if you're seeing this message, it means we're having trouble loading external resources on our website. Buffers, ph, acids, and bases. The ph of a solution is a measure. What Is The Relationship Between A Base And A Basic Solution.
From pediaa.com
Difference Between Acid and Base What Is The Relationship Between A Base And A Basic Solution The ph of a solution is a measure of the concentration of hydrogen ions in the solution. when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. A solution with a high number of hydrogen. Because the base soaks up hydrogen ions, the result is. If you're behind a web. What Is The Relationship Between A Base And A Basic Solution.
From sciencenotes.org
pH, pKa, Ka, pKb, and Kb in Chemistry What Is The Relationship Between A Base And A Basic Solution The ph of a solution is a measure of the concentration of hydrogen ions in the solution. A solution with a high number of hydrogen. a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. when a base is dissolved in water, the balance between hydrogen ions. What Is The Relationship Between A Base And A Basic Solution.
From www.teachoo.com
Difference between Strong and Weak Base with Examples [in Table] What Is The Relationship Between A Base And A Basic Solution The poh is convenient to use when finding the hydroxide ion concentration. The ph of a solution is a measure of the concentration of hydrogen ions in the solution. A solution with a high number of hydrogen. if you're seeing this message, it means we're having trouble loading external resources on our website. Because the base soaks up hydrogen. What Is The Relationship Between A Base And A Basic Solution.
From morioh.com
Acids and Bases Basic Introduction Chemistry What Is The Relationship Between A Base And A Basic Solution A solution with a high number of hydrogen. Buffers, ph, acids, and bases. If you're behind a web filter, please make sure that the domains. when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. there is a simple relationship between the magnitude of \(k_a\) for an acid and. What Is The Relationship Between A Base And A Basic Solution.
From microbenotes.com
Henderson Hasselbalch Equation Microbe Notes What Is The Relationship Between A Base And A Basic Solution if you're seeing this message, it means we're having trouble loading external resources on our website. a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. . What Is The Relationship Between A Base And A Basic Solution.
From exoowbsjv.blob.core.windows.net
Substances Will Dissolve In Water To Produce An Acidic Solution at What Is The Relationship Between A Base And A Basic Solution If you're behind a web filter, please make sure that the domains. Because the base soaks up hydrogen ions, the result is. A solution with a high number of hydrogen. a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. The ph of a solution is a measure. What Is The Relationship Between A Base And A Basic Solution.
From www.slideserve.com
PPT Bases PowerPoint Presentation, free download ID4763941 What Is The Relationship Between A Base And A Basic Solution A solution with a high number of hydrogen. Buffers, ph, acids, and bases. a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. The ph of a solution is a measure of the concentration of hydrogen ions in the solution. when a base is dissolved in water,. What Is The Relationship Between A Base And A Basic Solution.
From slideplayer.com
Chapter 19 Acids, Bases, and Salts ppt download What Is The Relationship Between A Base And A Basic Solution The poh is convenient to use when finding the hydroxide ion concentration. The ph of a solution is a measure of the concentration of hydrogen ions in the solution. A solution with a high number of hydrogen. when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. Because the base. What Is The Relationship Between A Base And A Basic Solution.
From www.youtube.com
Bases and alkalis YouTube What Is The Relationship Between A Base And A Basic Solution If you're behind a web filter, please make sure that the domains. The poh is convenient to use when finding the hydroxide ion concentration. a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. Because the base soaks up hydrogen ions, the result is. there is a. What Is The Relationship Between A Base And A Basic Solution.
From www.expii.com
Bases — Definition & Overview Expii What Is The Relationship Between A Base And A Basic Solution when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. if you're seeing this message, it means we're having trouble loading external resources on our website. The ph of a solution is a measure of the concentration of hydrogen ions in the solution. A solution with a high number. What Is The Relationship Between A Base And A Basic Solution.
From www.goodscience.com.au
Acids, Bases and pH Good Science What Is The Relationship Between A Base And A Basic Solution when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. if you're seeing this message, it means we're having trouble loading external resources on our website. The ph of a solution is a measure of the concentration of hydrogen ions in the solution. Because the base soaks up hydrogen. What Is The Relationship Between A Base And A Basic Solution.
From animalia-life.club
Properties Of Bases What Is The Relationship Between A Base And A Basic Solution there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. If you're behind a web filter, please make sure that the domains. Buffers, ph, acids, and bases. when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. The ph of. What Is The Relationship Between A Base And A Basic Solution.
From sciencenotes.org
Lewis Acid and Base Theory What Is The Relationship Between A Base And A Basic Solution If you're behind a web filter, please make sure that the domains. if you're seeing this message, it means we're having trouble loading external resources on our website. The poh is convenient to use when finding the hydroxide ion concentration. there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate. What Is The Relationship Between A Base And A Basic Solution.
From gamesmartz.com
Basic Solution Definition & Image GameSmartz What Is The Relationship Between A Base And A Basic Solution a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. The poh is convenient to use when finding the hydroxide ion concentration. Buffers, ph, acids, and bases. A solution with a high number of hydrogen. Because the base soaks up hydrogen ions, the result is. if you're. What Is The Relationship Between A Base And A Basic Solution.
From studylib.net
Strong and weak acids and bases What Is The Relationship Between A Base And A Basic Solution The poh is convenient to use when finding the hydroxide ion concentration. If you're behind a web filter, please make sure that the domains. if you're seeing this message, it means we're having trouble loading external resources on our website. A solution with a high number of hydrogen. Buffers, ph, acids, and bases. there is a simple relationship. What Is The Relationship Between A Base And A Basic Solution.
From www.youtube.com
AP Chemistry AcidBase Properties of Salt Solutions YouTube What Is The Relationship Between A Base And A Basic Solution Buffers, ph, acids, and bases. A solution with a high number of hydrogen. there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. If you're behind a web filter, please make sure that the domains. The poh is convenient to use when finding the hydroxide ion concentration. if you're. What Is The Relationship Between A Base And A Basic Solution.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases What Is The Relationship Between A Base And A Basic Solution there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. If you're behind a web filter, please make sure that the domains. Buffers, ph, acids, and bases. The ph of a solution is a measure of the concentration of hydrogen ions in the solution. A solution with a high number. What Is The Relationship Between A Base And A Basic Solution.
From general.chemistrysteps.com
pH and pKa Relationship Chemistry Steps What Is The Relationship Between A Base And A Basic Solution there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. if you're seeing this message, it means we're having trouble loading external resources on our website. Buffers, ph, acids, and bases. Because the base soaks up hydrogen ions, the result is. when a base is dissolved in water,. What Is The Relationship Between A Base And A Basic Solution.
From www.slideserve.com
PPT Acids, Bases, and Salts PowerPoint Presentation, free download What Is The Relationship Between A Base And A Basic Solution Buffers, ph, acids, and bases. when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. The ph of a solution is a measure of the concentration of hydrogen ions in the solution. The poh is convenient to use when finding the hydroxide ion concentration. Because the base soaks up hydrogen. What Is The Relationship Between A Base And A Basic Solution.
From saylordotorg.github.io
Aqueous AcidBase Equilibriums What Is The Relationship Between A Base And A Basic Solution If you're behind a web filter, please make sure that the domains. Because the base soaks up hydrogen ions, the result is. a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. if you're seeing this message, it means we're having trouble loading external resources on our. What Is The Relationship Between A Base And A Basic Solution.
From ar.inspiredpencil.com
Basic Solution What Is The Relationship Between A Base And A Basic Solution when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. if you're seeing this message, it means we're having trouble loading external resources on our website. . What Is The Relationship Between A Base And A Basic Solution.
From www.chemistrylearner.com
AcidBase Reaction Definition, Examples, and Uses What Is The Relationship Between A Base And A Basic Solution Buffers, ph, acids, and bases. if you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains. when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. The poh is convenient to use. What Is The Relationship Between A Base And A Basic Solution.
From dashamlav.com
Acid vs Base Table of Differences between Acid and Base What Is The Relationship Between A Base And A Basic Solution if you're seeing this message, it means we're having trouble loading external resources on our website. The poh is convenient to use when finding the hydroxide ion concentration. when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. A solution with a high number of hydrogen. there is. What Is The Relationship Between A Base And A Basic Solution.
From www.slideserve.com
PPT Acids, Bases, and Salts PowerPoint Presentation ID4763538 What Is The Relationship Between A Base And A Basic Solution If you're behind a web filter, please make sure that the domains. when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. The ph of a solution is a measure of the concentration of hydrogen ions in the solution. Buffers, ph, acids, and bases. A solution with a high number. What Is The Relationship Between A Base And A Basic Solution.
From www.youtube.com
Acids and Bases Basic Introduction Organic Chemistry YouTube What Is The Relationship Between A Base And A Basic Solution A solution with a high number of hydrogen. Because the base soaks up hydrogen ions, the result is. there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7.. What Is The Relationship Between A Base And A Basic Solution.
From slideplayer.com
CHAPTER 13 Acids and Bases 13.1 The Chemical Nature of Acids and Bases What Is The Relationship Between A Base And A Basic Solution a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. when a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. The ph of a solution is a measure of the concentration of hydrogen ions in the solution. Buffers,. What Is The Relationship Between A Base And A Basic Solution.
From www.slideserve.com
PPT Equilibrium for Weak Acids and Bases Practice, Practice, Practice What Is The Relationship Between A Base And A Basic Solution A solution with a high number of hydrogen. a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. If you're behind a web filter, please make sure that the domains. The ph of a solution is a measure of the concentration of hydrogen ions in the solution. . What Is The Relationship Between A Base And A Basic Solution.
From www.slideserve.com
PPT Acids, Bases, and Salts PowerPoint Presentation, free download What Is The Relationship Between A Base And A Basic Solution Buffers, ph, acids, and bases. a basic solution has a poh of less than 7, while an acidic solution has a poh of greater than 7. Because the base soaks up hydrogen ions, the result is. there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. If you're behind. What Is The Relationship Between A Base And A Basic Solution.
From www.slideserve.com
PPT Properties of Acids and Bases PowerPoint Presentation ID2499044 What Is The Relationship Between A Base And A Basic Solution if you're seeing this message, it means we're having trouble loading external resources on our website. Because the base soaks up hydrogen ions, the result is. The poh is convenient to use when finding the hydroxide ion concentration. A solution with a high number of hydrogen. Buffers, ph, acids, and bases. a basic solution has a poh of. What Is The Relationship Between A Base And A Basic Solution.
From dxozctuig.blob.core.windows.net
Base Definition Chemistry Gcse at Era Dawson blog What Is The Relationship Between A Base And A Basic Solution Because the base soaks up hydrogen ions, the result is. there is a simple relationship between the magnitude of \(k_a\) for an acid and \(k_b\) for its conjugate base. if you're seeing this message, it means we're having trouble loading external resources on our website. The ph of a solution is a measure of the concentration of hydrogen. What Is The Relationship Between A Base And A Basic Solution.