Aluminum Hydroxide Dissociation . In a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? Write a balanced dissociation equation. Aluminum hydroxide is an inorganic salt used as an antacid. The current status of aluminum behavior in alkaline solutions, including the hydroxide equilibrium solid phases and the. In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of molecules ionize, producing few. Aluminium hydroxide tends to form gelatinous precipitate that easily adsorbs various ions (this property is actually utilized in waste water treatment and. Al 2 (so 4) 3. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and (b) base. It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few ions, and. Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o, is amphoteric. Dissolution reactions in acid and base.
from www.slideshare.net
In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few ions, and. The current status of aluminum behavior in alkaline solutions, including the hydroxide equilibrium solid phases and the. Dissolution reactions in acid and base. Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o, is amphoteric. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and (b) base. Aluminium hydroxide tends to form gelatinous precipitate that easily adsorbs various ions (this property is actually utilized in waste water treatment and. In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of molecules ionize, producing few. Al 2 (so 4) 3. Aluminum hydroxide is an inorganic salt used as an antacid. In a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion?
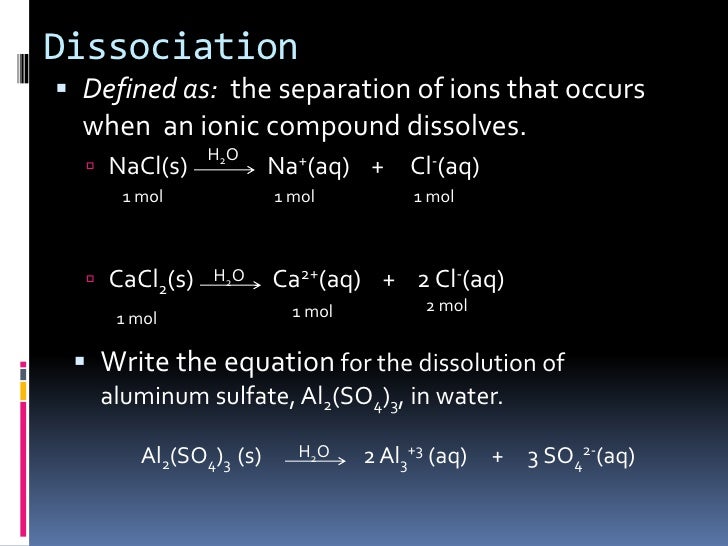
Chapter 14.1 Compounds in Aqueous Solutions
Aluminum Hydroxide Dissociation It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few ions, and. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and (b) base. Aluminum hydroxide is an inorganic salt used as an antacid. Al 2 (so 4) 3. Write a balanced dissociation equation. Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o, is amphoteric. Aluminium hydroxide tends to form gelatinous precipitate that easily adsorbs various ions (this property is actually utilized in waste water treatment and. In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of molecules ionize, producing few. Dissolution reactions in acid and base. It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. In a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? The current status of aluminum behavior in alkaline solutions, including the hydroxide equilibrium solid phases and the.
From www.numerade.com
SOLVED Calculate the [H+] for a 0.0628 M solution of aluminum hydroxide, Al(OH)3assuming Aluminum Hydroxide Dissociation Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o, is amphoteric. In a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? The current status of aluminum behavior in alkaline solutions, including the hydroxide equilibrium solid phases and the. Write a balanced dissociation equation. It is a basic compound that. Aluminum Hydroxide Dissociation.
From www.alamy.com
Formation of aluminium hydroxide precipitate by adding sodium hydroxide to a solution containing Aluminum Hydroxide Dissociation Dissolution reactions in acid and base. In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of molecules ionize, producing few. In a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? Aluminium hydroxide tends to form gelatinous precipitate that easily adsorbs various ions (this property is actually. Aluminum Hydroxide Dissociation.
From aluminumhydroxidesaidan.blogspot.com
Aluminum Hydroxide Aluminum Hydroxide Solubility In Water Aluminum Hydroxide Dissociation In a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? Aluminum hydroxide is an inorganic salt used as an antacid. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and (b) base. In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of. Aluminum Hydroxide Dissociation.
From www.coursehero.com
[Solved] For each chemical listed, write the dissociation / ionization... Course Hero Aluminum Hydroxide Dissociation Aluminum hydroxide is an inorganic salt used as an antacid. The current status of aluminum behavior in alkaline solutions, including the hydroxide equilibrium solid phases and the. Al 2 (so 4) 3. Write a balanced dissociation equation. Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o, is amphoteric. Aluminium hydroxide tends to form gelatinous. Aluminum Hydroxide Dissociation.
From www.youtube.com
Balance Al + H2O = Al(OH)3 + H2 (Aluminum and Water) YouTube Aluminum Hydroxide Dissociation It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o, is amphoteric. In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few ions, and. In a 0.23 mol/l aluminum. Aluminum Hydroxide Dissociation.
From www.youtube.com
How to Make Aluminum Hydroxide Al(OH)3 YouTube Aluminum Hydroxide Dissociation In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of molecules ionize, producing few. Al 2 (so 4) 3. The current status of aluminum behavior in alkaline solutions, including the hydroxide equilibrium solid phases and the. Aluminium hydroxide tends to form gelatinous precipitate that easily adsorbs various ions (this property is. Aluminum Hydroxide Dissociation.
From www.youtube.com
What happens when Sodium Hydroxide and Aluminium Reacts..? Exothermic Reaction YouTube Aluminum Hydroxide Dissociation In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few ions, and. Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o, is amphoteric. In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of. Aluminum Hydroxide Dissociation.
From www.youtube.com
HNO3+Al(OH)3=Al(NO3)3+H2O Balanced EquationNitric acid+Aluminum hydroxide=Aluminum nitrate Aluminum Hydroxide Dissociation Al 2 (so 4) 3. Aluminum hydroxide is an inorganic salt used as an antacid. It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and (b) base. Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o,. Aluminum Hydroxide Dissociation.
From www.chegg.com
Solved The formation constants for aluminum Aluminum Hydroxide Dissociation In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few ions, and. In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of molecules ionize, producing few. Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h. Aluminum Hydroxide Dissociation.
From www.numerade.com
SOLVED Aluminum hydroxide can be produced by the following reaction in water AlClz(aq) + 3Hz0 Aluminum Hydroxide Dissociation Aluminum hydroxide is an inorganic salt used as an antacid. Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o, is amphoteric. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and (b) base. It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. The current status. Aluminum Hydroxide Dissociation.
From www.toppr.com
Which of the following equation represents action of alkali on aluminium hydroxide? Aluminum Hydroxide Dissociation Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o, is amphoteric. In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of molecules ionize, producing few. It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. Write a balanced dissociation equation. Aluminium. Aluminum Hydroxide Dissociation.
From www.toppr.com
When aluminium hydroxide dissolves in NaOH solution, the product is Aluminum Hydroxide Dissociation In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few ions, and. Aluminium hydroxide tends to form gelatinous precipitate that easily adsorbs various ions (this property is actually utilized in waste water treatment and. It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions.. Aluminum Hydroxide Dissociation.
From pediaa.com
Difference Between Sodium Hydroxide and Aluminum Hydroxide Definition, Chemical Properties Aluminum Hydroxide Dissociation In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few ions, and. In a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o, is amphoteric. In the case of. Aluminum Hydroxide Dissociation.
From www.numerade.com
SOLVED Calculate the pH of 2.00x10^3 M aluminum hydroxide. Assume 100 percent dissociation. Aluminum Hydroxide Dissociation In a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? Aluminum hydroxide is an inorganic salt used as an antacid. In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of molecules ionize, producing few. It is a basic compound that acts by neutralizing hydrochloric acid in. Aluminum Hydroxide Dissociation.
From hxebyaqlb.blob.core.windows.net
Aluminum Hydroxide Dissociation Equation at Catherine Nevarez blog Aluminum Hydroxide Dissociation Write a balanced dissociation equation. In a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and (b) base. Al 2 (so 4) 3. Aluminium hydroxide tends to form. Aluminum Hydroxide Dissociation.
From www.researchgate.net
(PDF) Precipitation of aluminum hydroxide from sodium aluminate, by treatment with formalin, and Aluminum Hydroxide Dissociation It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. The current status of aluminum behavior in alkaline solutions, including the hydroxide equilibrium solid phases and the. Write a balanced dissociation equation. In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of molecules ionize, producing few. Aluminum. Aluminum Hydroxide Dissociation.
From www.scribd.com
Aluminum PDF Aluminium Hydroxide Aluminum Hydroxide Dissociation Dissolution reactions in acid and base. In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of molecules ionize, producing few. Aluminum hydroxide is an inorganic salt used as an antacid. It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. Aluminum hydroxide, written as either al(oh) 3. Aluminum Hydroxide Dissociation.
From www.slideshare.net
Chapter 14.1 Compounds in Aqueous Solutions Aluminum Hydroxide Dissociation Write a balanced dissociation equation. Dissolution reactions in acid and base. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and (b) base. In a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? Aluminium hydroxide tends to form gelatinous precipitate that easily adsorbs various ions (this property is actually utilized in. Aluminum Hydroxide Dissociation.
From hxebyaqlb.blob.core.windows.net
Aluminum Hydroxide Dissociation Equation at Catherine Nevarez blog Aluminum Hydroxide Dissociation Aluminum hydroxide is an inorganic salt used as an antacid. In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few ions, and. In a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? Dissolution reactions in acid and base. Write a balanced dissociation equation.. Aluminum Hydroxide Dissociation.
From aluminumhydroxidesaidan.blogspot.com
Aluminum Hydroxide Balanced Equation For Aluminum Hydroxide And Hydrochloric Acid Aluminum Hydroxide Dissociation Aluminum hydroxide is an inorganic salt used as an antacid. Dissolution reactions in acid and base. The current status of aluminum behavior in alkaline solutions, including the hydroxide equilibrium solid phases and the. Write a balanced dissociation equation. In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few. Aluminum Hydroxide Dissociation.
From www.youtube.com
Aluminum Hydroxide Precipitation YouTube Aluminum Hydroxide Dissociation Al 2 (so 4) 3. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and (b) base. Dissolution reactions in acid and base. In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of molecules ionize, producing few. Aluminium hydroxide tends to form gelatinous precipitate that easily adsorbs. Aluminum Hydroxide Dissociation.
From www.researchgate.net
Example aluminium hydroxide and hydroxyaluminosilicate structures... Download Scientific Diagram Aluminum Hydroxide Dissociation Aluminum hydroxide is an inorganic salt used as an antacid. In a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few ions, and. Al 2 (so 4) 3. Dissolution reactions in acid and base.. Aluminum Hydroxide Dissociation.
From www.museoinclusivo.com
What is the Formula for Aluminum Hydroxide? Exploring its Components and Chemistry Aluminum Aluminum Hydroxide Dissociation In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of molecules ionize, producing few. It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o, is amphoteric. The current status of aluminum behavior. Aluminum Hydroxide Dissociation.
From www.chegg.com
Solved 3. Aluminum hydroxide is insoluble in water (Kap Aluminum Hydroxide Dissociation In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of molecules ionize, producing few. Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o, is amphoteric. The current status of aluminum behavior in alkaline solutions, including the hydroxide equilibrium solid phases and the. Write chemical equations. Aluminum Hydroxide Dissociation.
From testbook.com
Aluminium Hydroxide Learn structure, preparation, properties Aluminum Hydroxide Dissociation Write a balanced dissociation equation. Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o, is amphoteric. In a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? Al 2 (so 4) 3. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and (b) base. It is. Aluminum Hydroxide Dissociation.
From www.youtube.com
Calculating the Solubility of Aluminum Hydroxide Test Boost for AP Chemistry Exam YouTube Aluminum Hydroxide Dissociation Al 2 (so 4) 3. Write a balanced dissociation equation. Aluminium hydroxide tends to form gelatinous precipitate that easily adsorbs various ions (this property is actually utilized in waste water treatment and. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and (b) base. Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3. Aluminum Hydroxide Dissociation.
From www.slideserve.com
PPT HelloOo…. PowerPoint Presentation ID2068170 Aluminum Hydroxide Dissociation In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few ions, and. Write a balanced dissociation equation. In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of molecules ionize, producing few. Aluminum hydroxide is an inorganic salt used as. Aluminum Hydroxide Dissociation.
From achs-prod.acs.org
Formation of Aluminum HydroxideDoped Surface Passivating Layers on Pyrite for Acid Rock Aluminum Hydroxide Dissociation The current status of aluminum behavior in alkaline solutions, including the hydroxide equilibrium solid phases and the. Aluminum hydroxide is an inorganic salt used as an antacid. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and (b) base. In a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? Al 2. Aluminum Hydroxide Dissociation.
From aluminumhydroxidesaidan.blogspot.com
Aluminum Hydroxide Standard Formation Reaction Of Solid Aluminum Hydroxide Aluminum Hydroxide Dissociation It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. Dissolution reactions in acid and base. Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o, is amphoteric. In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few ions,. Aluminum Hydroxide Dissociation.
From www.numerade.com
SOLVED Write the equation for the dissociation of aluminum hydroxide Al(OH)3. Calculate the Aluminum Hydroxide Dissociation Dissolution reactions in acid and base. In the case of a weak base, such as aluminum hydroxide, al (oh) 3, only a small percent of molecules ionize, producing few. The current status of aluminum behavior in alkaline solutions, including the hydroxide equilibrium solid phases and the. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and. Aluminum Hydroxide Dissociation.
From chempedia.info
Aluminum hydroxide reactions Big Chemical Encyclopedia Aluminum Hydroxide Dissociation In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few ions, and. Aluminum hydroxide, written as either al(oh) 3 or al 2 o 3 •3h 2 o, is amphoteric. Write a balanced dissociation equation. It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions.. Aluminum Hydroxide Dissociation.
From www.researchgate.net
Example aluminium hydroxide and hydroxyaluminosilicate structures... Download Scientific Diagram Aluminum Hydroxide Dissociation Aluminum hydroxide is an inorganic salt used as an antacid. Aluminium hydroxide tends to form gelatinous precipitate that easily adsorbs various ions (this property is actually utilized in waste water treatment and. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and (b) base. It is a basic compound that acts by neutralizing hydrochloric acid in. Aluminum Hydroxide Dissociation.
From www.scribd.com
Aluminium Hydroxide PDF Hydroxide Aluminium Oxide Aluminum Hydroxide Dissociation The current status of aluminum behavior in alkaline solutions, including the hydroxide equilibrium solid phases and the. Aluminium hydroxide tends to form gelatinous precipitate that easily adsorbs various ions (this property is actually utilized in waste water treatment and. It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. Write a balanced dissociation equation. Write chemical. Aluminum Hydroxide Dissociation.
From quynhiebear.blogspot.com
Write The Equation For The Dissociation Of Aluminum Hydroxide 31+ Pages Summary in Google Sheet Aluminum Hydroxide Dissociation Dissolution reactions in acid and base. Write chemical equations to describe the dissolution of aluminum hydroxide in (a) acid and (b) base. In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few ions, and. Al 2 (so 4) 3. Aluminium hydroxide tends to form gelatinous precipitate that easily. Aluminum Hydroxide Dissociation.
From www.numerade.com
SOLVED If the pH of a solution of aluminum hydroxide is 9.85, what is the concentration of the Aluminum Hydroxide Dissociation It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. In the case of a weak base, such as aluminum hydroxide, al(oh) 3, only a small percent of molecules ionize, producing few ions, and. Aluminium hydroxide tends to form gelatinous precipitate that easily adsorbs various ions (this property is actually utilized in waste water treatment and.. Aluminum Hydroxide Dissociation.