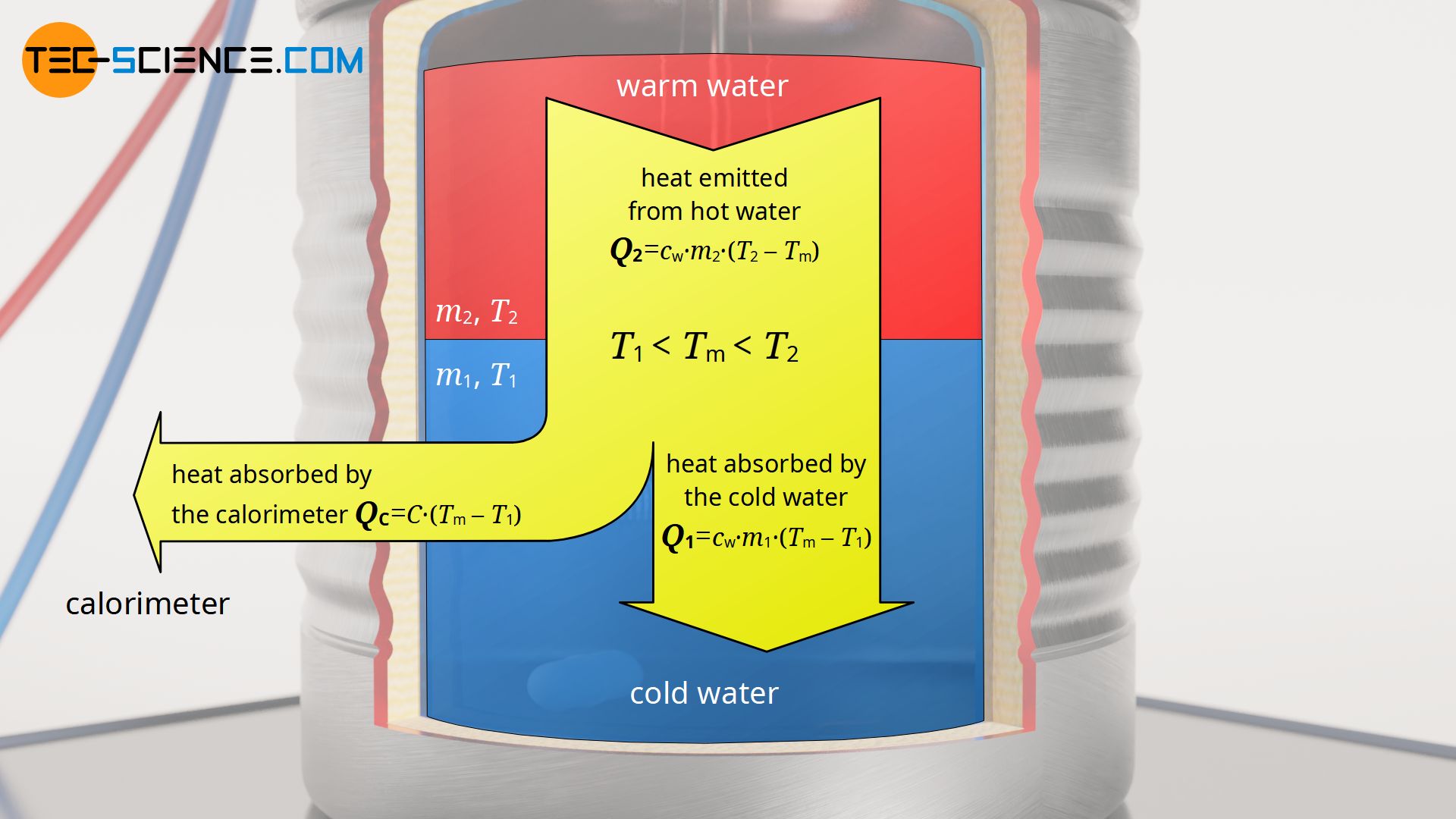
How Does A Calorimeter Measure Specific Heat . To determine the specific heat of an unknown metal, place a heated piece of the metal in water in the inner vessel of the calorimeter. Once you have measured the final. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the calorimeter to the heat lost/absorbed by the reaction. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Calorimetry is used to measure amounts of heat transferred to. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is used to measure amounts of heat transferred to. The specific heat is numerically equal to the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry.
from www.tec-science.com
One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To determine the specific heat of an unknown metal, place a heated piece of the metal in water in the inner vessel of the calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to. Once you have measured the final. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Calorimetry is used to measure amounts of heat transferred to. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is used to measure amounts of heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry.
Calorimeter to determine the specific heat capacities of liquids tec
How Does A Calorimeter Measure Specific Heat One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. To determine the specific heat of an unknown metal, place a heated piece of the metal in water in the inner vessel of the calorimeter. Calorimetry is used to measure amounts of heat transferred to. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the calorimeter to the heat lost/absorbed by the reaction. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to. Once you have measured the final. The specific heat is numerically equal to the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 How Does A Calorimeter Measure Specific Heat Calorimetry is used to measure amounts of heat transferred to. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat. Calorimetry is used to measure amounts of heat transferred to. To determine the specific heat of an unknown metal, place. How Does A Calorimeter Measure Specific Heat.
From pt.wikihow.com
Como Calcular o Calor Específico 6 Passos Imagens) How Does A Calorimeter Measure Specific Heat Calorimetry is used to measure amounts of heat transferred to. Calorimetry is used to measure amounts of heat transferred to. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To determine the specific heat of an unknown metal, place a heated piece of the metal in water. How Does A Calorimeter Measure Specific Heat.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter How Does A Calorimeter Measure Specific Heat To determine the specific heat of an unknown metal, place a heated piece of the metal in water in the inner vessel of the calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in. How Does A Calorimeter Measure Specific Heat.
From pressbooks.online.ucf.edu
1.4 Heat Transfer, Specific Heat, and Calorimetry General Physics How Does A Calorimeter Measure Specific Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is used to measure amounts of heat transferred to. Calorimetry is used to measure amounts of heat transferred to. Calorimetry is used to measure. How Does A Calorimeter Measure Specific Heat.
From chemistrytalk.org
Calorimetry ChemTalk How Does A Calorimeter Measure Specific Heat One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry measures enthalpy changes during chemical. How Does A Calorimeter Measure Specific Heat.
From printablezonemarrow.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry How Does A Calorimeter Measure Specific Heat Calorimetry is used to measure amounts of heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry measures enthalpy changes during chemical processes, where. How Does A Calorimeter Measure Specific Heat.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6133898 How Does A Calorimeter Measure Specific Heat The specific heat is numerically equal to the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to. Once you have measured the final. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. How Does A Calorimeter Measure Specific Heat.
From www.slideshare.net
Lesson Enthalpy and Calorimetry How Does A Calorimeter Measure Specific Heat Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat. Once you have measured the final. One technique we can use to measure the amount. How Does A Calorimeter Measure Specific Heat.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo How Does A Calorimeter Measure Specific Heat One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Calorimetry is used to measure amounts of heat.. How Does A Calorimeter Measure Specific Heat.
From learningcampusscarf.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry How Does A Calorimeter Measure Specific Heat To determine the specific heat of an unknown metal, place a heated piece of the metal in water in the inner vessel of the calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature. How Does A Calorimeter Measure Specific Heat.
From studylib.net
Chapter 5b (PowerPoint) How Does A Calorimeter Measure Specific Heat One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or. How Does A Calorimeter Measure Specific Heat.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts How Does A Calorimeter Measure Specific Heat Calorimetry is used to measure amounts of heat transferred to. Calorimetry is used to measure amounts of heat transferred to. Calorimetry is used to measure amounts of heat. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the calorimeter to the heat lost/absorbed by the reaction. One technique we can use to measure. How Does A Calorimeter Measure Specific Heat.
From www.thoughtco.com
Calorimeter Definition in Chemistry How Does A Calorimeter Measure Specific Heat Calorimetry is used to measure amounts of heat. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Calorimetry is used to measure amounts of heat transferred to. Calorimetry is used to measure amounts of heat transferred to. One technique we can use to measure the amount of heat involved. How Does A Calorimeter Measure Specific Heat.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube How Does A Calorimeter Measure Specific Heat To determine the specific heat of an unknown metal, place a heated piece of the metal in water in the inner vessel of the calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the. How Does A Calorimeter Measure Specific Heat.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec How Does A Calorimeter Measure Specific Heat One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To determine the specific heat of an unknown metal, place a heated piece of the metal in water in the inner vessel of the calorimeter. The symbol c stands for the specific heat (also called “specific heat capacity”). How Does A Calorimeter Measure Specific Heat.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog How Does A Calorimeter Measure Specific Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Once you have measured the final. Calorimetry is used to measure amounts of heat transferred to. To determine. How Does A Calorimeter Measure Specific Heat.
From keystagewiki.com
GCSE Physics Required Practical Determining Specific Heat Capacity How Does A Calorimeter Measure Specific Heat One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to. Once you have measured the final. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the calorimeter to the heat lost/absorbed. How Does A Calorimeter Measure Specific Heat.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow How Does A Calorimeter Measure Specific Heat One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the calorimeter to the heat lost/absorbed by the reaction. Calorimetry measures enthalpy changes during. How Does A Calorimeter Measure Specific Heat.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec How Does A Calorimeter Measure Specific Heat Calorimetry is used to measure amounts of heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The specific heat is numerically equal to the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry.. How Does A Calorimeter Measure Specific Heat.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube How Does A Calorimeter Measure Specific Heat To determine the specific heat of an unknown metal, place a heated piece of the metal in water in the inner vessel of the calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in. How Does A Calorimeter Measure Specific Heat.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download How Does A Calorimeter Measure Specific Heat Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the calorimeter to the heat lost/absorbed by the reaction. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in. How Does A Calorimeter Measure Specific Heat.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors How Does A Calorimeter Measure Specific Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat. One technique we can use to measure the amount of. How Does A Calorimeter Measure Specific Heat.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube How Does A Calorimeter Measure Specific Heat One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. To determine the. How Does A Calorimeter Measure Specific Heat.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates How Does A Calorimeter Measure Specific Heat Calorimetry is used to measure amounts of heat transferred to. To determine the specific heat of an unknown metal, place a heated piece of the metal in water in the inner vessel of the calorimeter. Once you have measured the final. One technique we can use to measure the amount of heat involved in a chemical or physical process is. How Does A Calorimeter Measure Specific Heat.
From sites.google.com
Calorimetry Preliminary HSC Chemistry How Does A Calorimeter Measure Specific Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The specific heat is numerically equal to the. Calorimetry is used to measure amounts of heat transferred to.. How Does A Calorimeter Measure Specific Heat.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors How Does A Calorimeter Measure Specific Heat The specific heat is numerically equal to the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat. Once you have measured the final. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by. How Does A Calorimeter Measure Specific Heat.
From www.youtube.com
Calorimetry Calculate Specific Heat YouTube How Does A Calorimeter Measure Specific Heat Calorimetry is used to measure amounts of heat. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the calorimeter to the heat lost/absorbed by the reaction. To determine the specific heat of an unknown metal, place a heated piece of the metal in water in the inner vessel of the calorimeter. One technique. How Does A Calorimeter Measure Specific Heat.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec How Does A Calorimeter Measure Specific Heat To determine the specific heat of an unknown metal, place a heated piece of the metal in water in the inner vessel of the calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The specific heat is numerically equal to the. Once you have measured the. How Does A Calorimeter Measure Specific Heat.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation How Does A Calorimeter Measure Specific Heat Calorimetry is used to measure amounts of heat transferred to. To determine the specific heat of an unknown metal, place a heated piece of the metal in water in the inner vessel of the calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we. How Does A Calorimeter Measure Specific Heat.
From deon-has-edwards.blogspot.com
Heat Capacity of Calorimeter DeonhasEdwards How Does A Calorimeter Measure Specific Heat To determine the specific heat of an unknown metal, place a heated piece of the metal in water in the inner vessel of the calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in. How Does A Calorimeter Measure Specific Heat.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica How Does A Calorimeter Measure Specific Heat Calorimetry is used to measure amounts of heat. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the calorimeter to the heat lost/absorbed by the reaction. Calorimetry is used to measure amounts of heat transferred to. One technique we can use to measure the amount of heat involved in a chemical or physical. How Does A Calorimeter Measure Specific Heat.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec How Does A Calorimeter Measure Specific Heat To determine the specific heat of an unknown metal, place a heated piece of the metal in water in the inner vessel of the calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Once you have measured the final. Calorimetry measures enthalpy changes during chemical processes,. How Does A Calorimeter Measure Specific Heat.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6655927 How Does A Calorimeter Measure Specific Heat Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is used to measure amounts of heat transferred to. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To determine the specific heat of an unknown metal, place a heated piece. How Does A Calorimeter Measure Specific Heat.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry How Does A Calorimeter Measure Specific Heat Calorimetry is used to measure amounts of heat transferred to. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the calorimeter to the heat lost/absorbed by the reaction. Calorimetry is used. How Does A Calorimeter Measure Specific Heat.
From www.numerade.com
SOLVED In the laboratory a "coffee cup calorimeter, Or constant How Does A Calorimeter Measure Specific Heat Calorimetry is used to measure amounts of heat transferred to. Calorimetry is used to measure amounts of heat. Calorimeters can also measure the molar enthalpy of reaction, by simply equating the heat absorbed/lost by the calorimeter to the heat lost/absorbed by the reaction. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the. How Does A Calorimeter Measure Specific Heat.