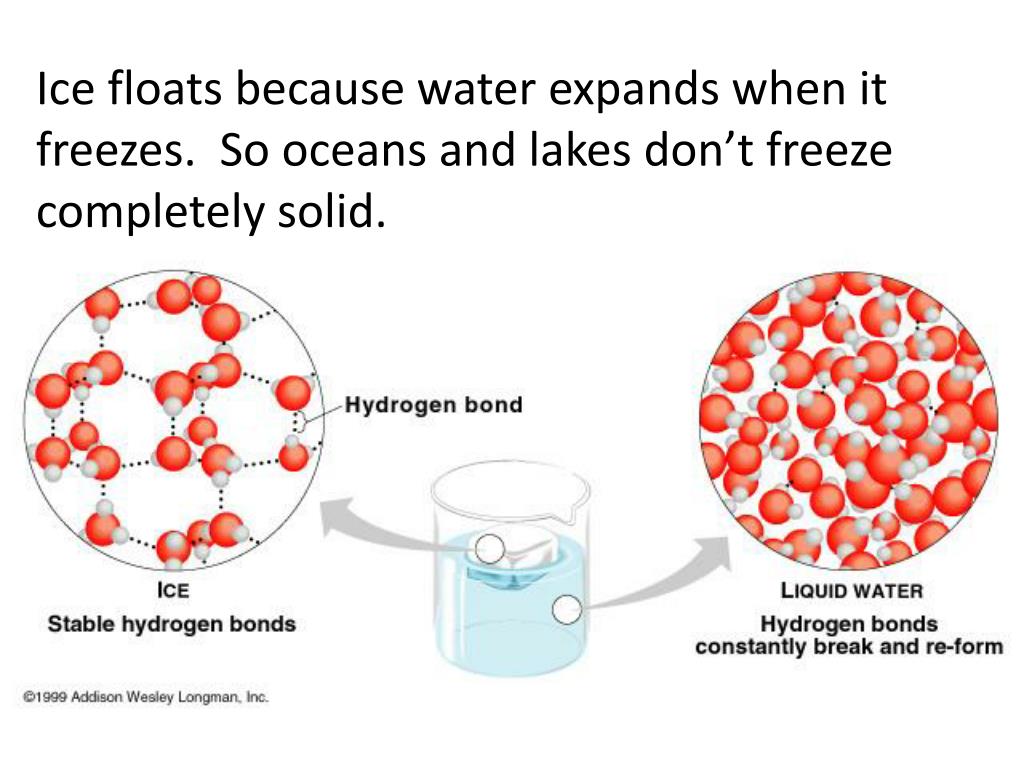
Why Does Ice Float On Water Give Scientific Reason . So, why does ice float on water? Something denser than water, like a rock, will sink. Scientists will tell you it has to do with density, which is a measure of mass per unit of volume. Most other substances, by contrast, become denser in the solid phase. We can explain this phenomenon with the help of a theory called archimedes' principle. The scientific explanation for why ice floats on water is based on the concept of density. What is the scientific explanation for why ice floats on water? Water ice, the solid state of water, floats because it is less dense than its liquid form. This is because the molecules in ice are further apart than the molecules in liquid water. As it reaches the solid state (ice), the hydrogen bonds become stable and arrange themselves in such a way that the adjacent oxygen atoms are spread far apart from each. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. This produces a crystal lattice commonly known as ice. Ice floats because it is about 9% less dense than liquid water.
from www.slideserve.com
Ice floats because it is less dense than the water. This produces a crystal lattice commonly known as ice. Ice floats because it is about 9% less dense than liquid water. This is because the molecules in ice are further apart than the molecules in liquid water. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. We can explain this phenomenon with the help of a theory called archimedes' principle. Most other substances, by contrast, become denser in the solid phase. The hydrogen bonds in ice are more stable and locked with a large number of empty spaces between them due to which density of molecules decreases. The molecules in ice are held further.
PPT Water, water everywhere! PowerPoint Presentation, free download
Why Does Ice Float On Water Give Scientific Reason In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. Ice is less dense than. Water ice, the solid state of water, floats because it is less dense than its liquid form. Most other substances, by contrast, become denser in the solid phase. An object less dense than water will float. What is the scientific explanation for why ice floats on water? Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. This is because the molecules in ice are further apart than the molecules in liquid water. So, why does ice float on water? As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. As it reaches the solid state (ice), the hydrogen bonds become stable and arrange themselves in such a way that the adjacent oxygen atoms are spread far apart from each. Ice floats because it is less dense than the water. Scientists will tell you it has to do with density, which is a measure of mass per unit of volume. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. Something denser than water, like a rock, will sink. Ice floats because it is about 9% less dense than liquid water.
From www.jagranjosh.com
What is the reason behind floating ice on water Why Does Ice Float On Water Give Scientific Reason Most other substances, by contrast, become denser in the solid phase. Ice floats because it is less dense than the water. An object less dense than water will float. Something denser than water, like a rock, will sink. As it reaches the solid state (ice), the hydrogen bonds become stable and arrange themselves in such a way that the adjacent. Why Does Ice Float On Water Give Scientific Reason.
From quizastrictive.z4.web.core.windows.net
Why Can Ice Float On Liquid Water Why Does Ice Float On Water Give Scientific Reason Ice is less dense than. This is because the molecules in ice are further apart than the molecules in liquid water. The molecules in ice are held further. We can explain this phenomenon with the help of a theory called archimedes' principle. As it reaches the solid state (ice), the hydrogen bonds become stable and arrange themselves in such a. Why Does Ice Float On Water Give Scientific Reason.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download Why Does Ice Float On Water Give Scientific Reason Most other substances, by contrast, become denser in the solid phase. The scientific explanation for why ice floats on water is based on the concept of density. As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. Something denser than water, like a rock, will sink. So, why does ice float. Why Does Ice Float On Water Give Scientific Reason.
From slidesharenow.blogspot.com
Why Does Ice Float On Water slideshare Why Does Ice Float On Water Give Scientific Reason This is because the molecules in ice are further apart than the molecules in liquid water. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. Most other substances, by contrast, become denser in the solid phase. Something denser than water, like a rock, will sink. So, why. Why Does Ice Float On Water Give Scientific Reason.
From www.youtube.com
Why does ice float in water? Zaidan and Charles Morton YouTube Why Does Ice Float On Water Give Scientific Reason The molecules in ice are held further. This produces a crystal lattice commonly known as ice. As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. Ice floats because it is about 9% less dense than liquid water. What is the scientific explanation for why ice floats on water? As it. Why Does Ice Float On Water Give Scientific Reason.
From www.youtube.com
Why does ice floats on water ? QnA Explained YouTube Why Does Ice Float On Water Give Scientific Reason What is the scientific explanation for why ice floats on water? Most other substances, by contrast, become denser in the solid phase. This produces a crystal lattice commonly known as ice. Ice floats because it is less dense than the water. We can explain this phenomenon with the help of a theory called archimedes' principle. An object less dense than. Why Does Ice Float On Water Give Scientific Reason.
From slideplayer.com
Water Unit. ppt download Why Does Ice Float On Water Give Scientific Reason In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. An object less dense than water will float. The molecules in ice are held further. Scientists will tell you it has to do with density, which is a measure of mass per unit of volume. What is the. Why Does Ice Float On Water Give Scientific Reason.
From issuu.com
Why Does Ice Float On Water Chemistry? by AmericanLifeguardManual Issuu Why Does Ice Float On Water Give Scientific Reason In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. Something denser than water, like a rock, will sink. Water ice, the solid state of water, floats because it is less dense than its liquid form. An object less dense than water will float. What is the scientific. Why Does Ice Float On Water Give Scientific Reason.
From www.youtube.com
Why Does Ice Float On Water? YouTube Why Does Ice Float On Water Give Scientific Reason An object less dense than water will float. Ice is less dense than. Ice floats because it is about 9% less dense than liquid water. This produces a crystal lattice commonly known as ice. The molecules in ice are held further. Scientists will tell you it has to do with density, which is a measure of mass per unit of. Why Does Ice Float On Water Give Scientific Reason.
From www.youtube.com
Why Ice Floats on Water YouTube Why Does Ice Float On Water Give Scientific Reason Scientists will tell you it has to do with density, which is a measure of mass per unit of volume. We can explain this phenomenon with the help of a theory called archimedes' principle. As it reaches the solid state (ice), the hydrogen bonds become stable and arrange themselves in such a way that the adjacent oxygen atoms are spread. Why Does Ice Float On Water Give Scientific Reason.
From slidesharenow.blogspot.com
Why Does Ice Float On Water slideshare Why Does Ice Float On Water Give Scientific Reason This is because the molecules in ice are further apart than the molecules in liquid water. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. The hydrogen bonds in ice are more stable and locked with a large number of empty spaces between them due to which density. Why Does Ice Float On Water Give Scientific Reason.
From higheducations.com
Why Does Ice Float in Liquid Water? Why Does Ice Float On Water Give Scientific Reason The scientific explanation for why ice floats on water is based on the concept of density. Ice floats because it is about 9% less dense than liquid water. Most other substances, by contrast, become denser in the solid phase. We can explain this phenomenon with the help of a theory called archimedes' principle. Ice floats because it is less dense. Why Does Ice Float On Water Give Scientific Reason.
From thevigyan.com
Why Does Ice Float in Water? The Vigyan Why Does Ice Float On Water Give Scientific Reason Ice is less dense than. Most other substances, by contrast, become denser in the solid phase. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. The scientific explanation for why ice floats on water is based on the concept of density. Water ice, the solid state of water,. Why Does Ice Float On Water Give Scientific Reason.
From medium.com
Why Does Ice Float on Water? by Why Does That Science Medium Why Does Ice Float On Water Give Scientific Reason Scientists will tell you it has to do with density, which is a measure of mass per unit of volume. Ice is less dense than. Ice floats because it is less dense than the water. This produces a crystal lattice commonly known as ice. The scientific explanation for why ice floats on water is based on the concept of density.. Why Does Ice Float On Water Give Scientific Reason.
From slidesharenow.blogspot.com
Why Does Ice Float On Water slideshare Why Does Ice Float On Water Give Scientific Reason The hydrogen bonds in ice are more stable and locked with a large number of empty spaces between them due to which density of molecules decreases. The scientific explanation for why ice floats on water is based on the concept of density. Although ice is a solid form of water, ice is lighter than water because the ice is less. Why Does Ice Float On Water Give Scientific Reason.
From www.slideserve.com
PPT WHY? PowerPoint Presentation, free download ID2511404 Why Does Ice Float On Water Give Scientific Reason We can explain this phenomenon with the help of a theory called archimedes' principle. Most other substances, by contrast, become denser in the solid phase. This produces a crystal lattice commonly known as ice. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. An object less dense than. Why Does Ice Float On Water Give Scientific Reason.
From sealevel.nasa.gov
Melting Ocean Ice Affects Sea Level Unlike Ice Cubes in a Glass Why Does Ice Float On Water Give Scientific Reason Water ice, the solid state of water, floats because it is less dense than its liquid form. The hydrogen bonds in ice are more stable and locked with a large number of empty spaces between them due to which density of molecules decreases. What is the scientific explanation for why ice floats on water? Although ice is a solid form. Why Does Ice Float On Water Give Scientific Reason.
From www.slideserve.com
PPT Chapter 11 Fluid Statics PowerPoint Presentation, free download Why Does Ice Float On Water Give Scientific Reason The scientific explanation for why ice floats on water is based on the concept of density. So, why does ice float on water? This produces a crystal lattice commonly known as ice. Ice floats because it is less dense than the water. Although ice is a solid form of water, ice is lighter than water because the ice is less. Why Does Ice Float On Water Give Scientific Reason.
From www.pinterest.com
Scientific Explanation of Why Ice Floats Science for kids, Hydrogen Why Does Ice Float On Water Give Scientific Reason Ice floats because it is about 9% less dense than liquid water. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. So, why does ice float on water? Water ice, the solid state of water, floats because it is less dense than its liquid form. What is the. Why Does Ice Float On Water Give Scientific Reason.
From www.youtube.com
Why ice floats on water Class 9 Chapter Chemistry NCERT YouTube Why Does Ice Float On Water Give Scientific Reason Ice floats because it is less dense than the water. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. An object less dense than water will float. Ice floats because it is about 9% less dense than liquid water. Something denser than water, like a rock, will. Why Does Ice Float On Water Give Scientific Reason.
From www.dreamstime.com
Why Does Ice Float on Water Infographic Diagram Stock Vector Why Does Ice Float On Water Give Scientific Reason So, why does ice float on water? Ice floats because it is about 9% less dense than liquid water. Ice floats because it is less dense than the water. The hydrogen bonds in ice are more stable and locked with a large number of empty spaces between them due to which density of molecules decreases. Water ice, the solid state. Why Does Ice Float On Water Give Scientific Reason.
From www.slideserve.com
PPT Ch 1 The Chemistry of Life PowerPoint Presentation, free Why Does Ice Float On Water Give Scientific Reason An object less dense than water will float. Ice floats because it is less dense than the water. As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. Water ice, the solid state of water, floats because it is less dense than its liquid form. Ice floats because it is about. Why Does Ice Float On Water Give Scientific Reason.
From www.slideserve.com
PPT Water, water everywhere! PowerPoint Presentation, free download Why Does Ice Float On Water Give Scientific Reason Ice floats because it is less dense than the water. We can explain this phenomenon with the help of a theory called archimedes' principle. As it reaches the solid state (ice), the hydrogen bonds become stable and arrange themselves in such a way that the adjacent oxygen atoms are spread far apart from each. This is because the molecules in. Why Does Ice Float On Water Give Scientific Reason.
From www.slideshare.net
Why does ice float on water? Why Does Ice Float On Water Give Scientific Reason Scientists will tell you it has to do with density, which is a measure of mass per unit of volume. Something denser than water, like a rock, will sink. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. Although ice is a solid form of water, ice. Why Does Ice Float On Water Give Scientific Reason.
From academichelp.net
Why Does Ice Float on Water? The Science Behind It Explained Why Does Ice Float On Water Give Scientific Reason Scientists will tell you it has to do with density, which is a measure of mass per unit of volume. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. The hydrogen bonds in ice are more stable and locked with a large number of empty spaces between. Why Does Ice Float On Water Give Scientific Reason.
From www.teachoo.com
Liquids generally have lower density as compared to solids. But you Why Does Ice Float On Water Give Scientific Reason What is the scientific explanation for why ice floats on water? An object less dense than water will float. Ice floats because it is less dense than the water. This is because the molecules in ice are further apart than the molecules in liquid water. As it reaches the solid state (ice), the hydrogen bonds become stable and arrange themselves. Why Does Ice Float On Water Give Scientific Reason.
From techiescientist.com
Why Does Ice Float on Water? Techiescientist Why Does Ice Float On Water Give Scientific Reason Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. Something denser than water, like a rock, will sink. Water ice, the solid state of water, floats because it is less dense than its liquid form. This is because the molecules in ice are further apart than the molecules. Why Does Ice Float On Water Give Scientific Reason.
From www.slideserve.com
PPT L14 Fluids [3] PowerPoint Presentation, free download ID5631091 Why Does Ice Float On Water Give Scientific Reason This produces a crystal lattice commonly known as ice. As it reaches the solid state (ice), the hydrogen bonds become stable and arrange themselves in such a way that the adjacent oxygen atoms are spread far apart from each. Water ice, the solid state of water, floats because it is less dense than its liquid form. So, why does ice. Why Does Ice Float On Water Give Scientific Reason.
From www.slideserve.com
PPT Why Ice Floats PowerPoint Presentation, free download ID9719781 Why Does Ice Float On Water Give Scientific Reason The scientific explanation for why ice floats on water is based on the concept of density. So, why does ice float on water? Ice floats because it is less dense than the water. Scientists will tell you it has to do with density, which is a measure of mass per unit of volume. Most other substances, by contrast, become denser. Why Does Ice Float On Water Give Scientific Reason.
From omny.fm
Science Week Why does ice float on water? Bec and Norm Omny.fm Why Does Ice Float On Water Give Scientific Reason Ice floats because it is about 9% less dense than liquid water. Scientists will tell you it has to do with density, which is a measure of mass per unit of volume. Water ice, the solid state of water, floats because it is less dense than its liquid form. As it reaches the solid state (ice), the hydrogen bonds become. Why Does Ice Float On Water Give Scientific Reason.
From www.branchor.com
Why does ice float on water? Exploring the science and consequences Why Does Ice Float On Water Give Scientific Reason This produces a crystal lattice commonly known as ice. So, why does ice float on water? What is the scientific explanation for why ice floats on water? Ice floats because it is less dense than the water. The hydrogen bonds in ice are more stable and locked with a large number of empty spaces between them due to which density. Why Does Ice Float On Water Give Scientific Reason.
From vervetimes.com
The Reason Why Ice Floats Explained Live Science Verve times Why Does Ice Float On Water Give Scientific Reason We can explain this phenomenon with the help of a theory called archimedes' principle. This produces a crystal lattice commonly known as ice. Most other substances, by contrast, become denser in the solid phase. So, why does ice float on water? The scientific explanation for why ice floats on water is based on the concept of density. As the water. Why Does Ice Float On Water Give Scientific Reason.
From learnglassblowing.com
Why Ice Cubes Float In Water Learn Glass Blowing Why Does Ice Float On Water Give Scientific Reason So, why does ice float on water? We can explain this phenomenon with the help of a theory called archimedes' principle. Although ice is a solid form of water, ice is lighter than water because the ice is less dense than water. Most other substances, by contrast, become denser in the solid phase. Something denser than water, like a rock,. Why Does Ice Float On Water Give Scientific Reason.
From www.pinterest.com
Why does ice float in water? Zaidan and Charles Morton Why Does Ice Float On Water Give Scientific Reason This produces a crystal lattice commonly known as ice. What is the scientific explanation for why ice floats on water? Ice floats because it is about 9% less dense than liquid water. We can explain this phenomenon with the help of a theory called archimedes' principle. The hydrogen bonds in ice are more stable and locked with a large number. Why Does Ice Float On Water Give Scientific Reason.
From www.youtube.com
Why does ice float on water? Detailed Explanation YouTube Why Does Ice Float On Water Give Scientific Reason Ice is less dense than. This produces a crystal lattice commonly known as ice. Most other substances, by contrast, become denser in the solid phase. Scientists will tell you it has to do with density, which is a measure of mass per unit of volume. The scientific explanation for why ice floats on water is based on the concept of. Why Does Ice Float On Water Give Scientific Reason.