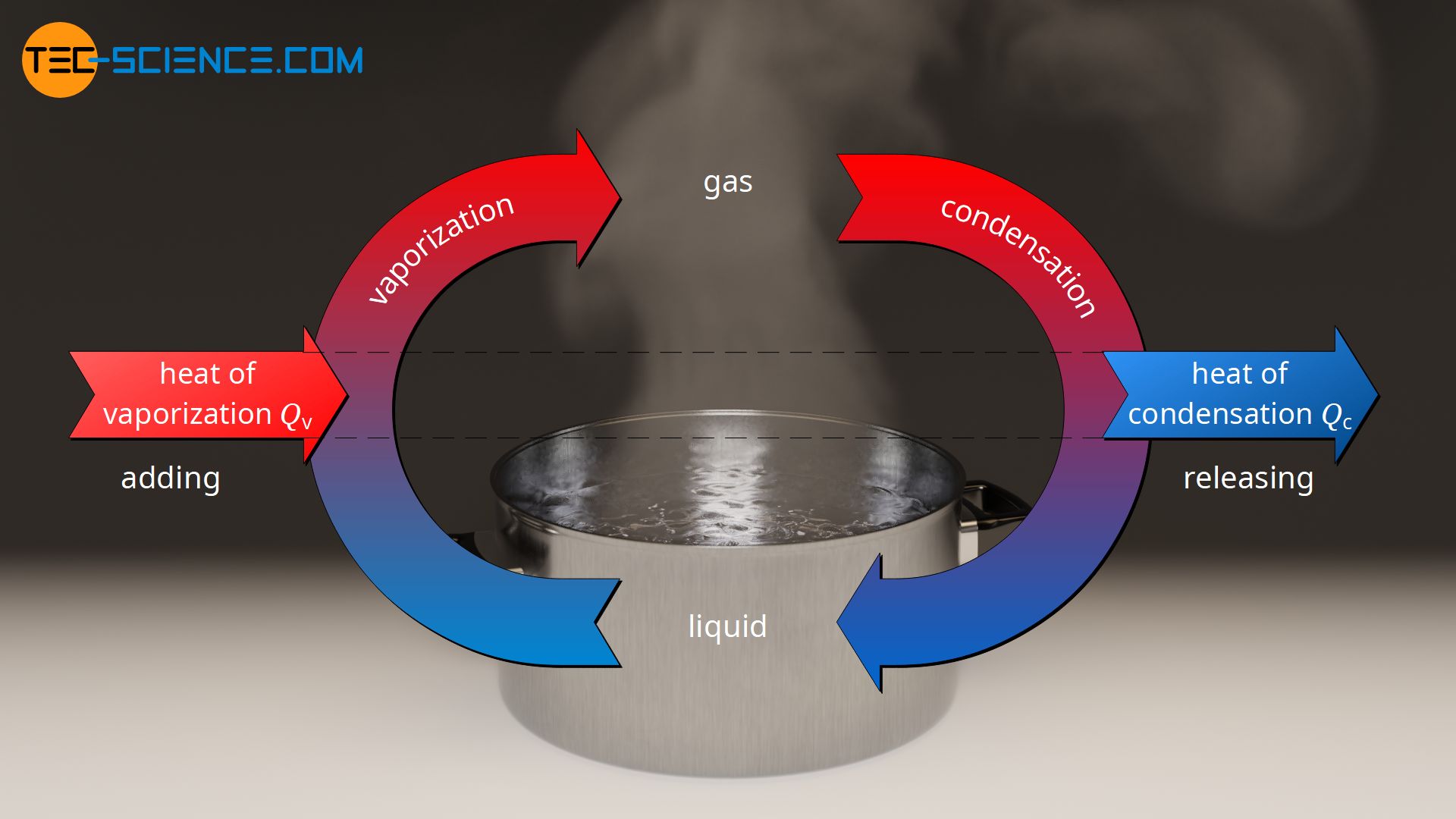
Condensed Gas Meaning . Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. It is the reverse of vaporization. During condensation, atoms and molecules form clusters. Condensation is the change in the state of matter from the gas phase to the liquid phase. For example, within a cloud, water nucleates around a dust, pollen, or microbial particle. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). Eventually the clusters reach sufficient mass to form droplets. Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that can condense from gas when the. Condensate gases are the result of the transition of a gaseous substance into a liquid state due to a drop in pressure or temperature.
from anyquotedistrictjibril.blogspot.com
Eventually the clusters reach sufficient mass to form droplets. For example, within a cloud, water nucleates around a dust, pollen, or microbial particle. It is the reverse of vaporization. Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that can condense from gas when the. Condensate gases are the result of the transition of a gaseous substance into a liquid state due to a drop in pressure or temperature. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. During condensation, atoms and molecules form clusters. Condensation is the change in the state of matter from the gas phase to the liquid phase.
The Best 9 What Is Condensation In Science anyquotedistrictjibril
Condensed Gas Meaning Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. Condensate gases are the result of the transition of a gaseous substance into a liquid state due to a drop in pressure or temperature. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). Condensation is the change in the state of matter from the gas phase to the liquid phase. Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that can condense from gas when the. Eventually the clusters reach sufficient mass to form droplets. For example, within a cloud, water nucleates around a dust, pollen, or microbial particle. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. It is the reverse of vaporization. During condensation, atoms and molecules form clusters.
From www.askdifference.com
Gases vs. Gasses — What’s the Difference? Condensed Gas Meaning During condensation, atoms and molecules form clusters. Condensate gases are the result of the transition of a gaseous substance into a liquid state due to a drop in pressure or temperature. It is the reverse of vaporization. Condensation is the change in the state of matter from the gas phase to the liquid phase. For example, within a cloud, water. Condensed Gas Meaning.
From www.thebusinessguardians.com
Gases Definition, Examples, List of the Elements, and More Condensed Gas Meaning Eventually the clusters reach sufficient mass to form droplets. Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that can condense from gas when the. During condensation, atoms and molecules form clusters. Condensation is the change in the state of matter from the gas phase to the liquid phase. At temperatures below 31°c (the critical. Condensed Gas Meaning.
From www.youtube.com
Gas Meaning Definition of Gas YouTube Condensed Gas Meaning Eventually the clusters reach sufficient mass to form droplets. Condensation is the change in the state of matter from the gas phase to the liquid phase. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. Condensate gases are the result of the transition of a gaseous. Condensed Gas Meaning.
From studylib.net
Condensed Phases Condensed Gas Meaning For example, within a cloud, water nucleates around a dust, pollen, or microbial particle. It is the reverse of vaporization. During condensation, atoms and molecules form clusters. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). Gas condensates are liquids, generally straight chain alkanes in the c2. Condensed Gas Meaning.
From slideplayer.com
EVAPORATION, CONDENSATION, PRECIPITATION AND TRANSPIRATION ppt download Condensed Gas Meaning Condensation is the change in the state of matter from the gas phase to the liquid phase. Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that can condense from gas when the. Condensate gases are the result of the transition of a gaseous substance into a liquid state due to a drop in pressure. Condensed Gas Meaning.
From www.youtube.com
Define Solid Liquid and Gas with examples \ Solid \ Liquid \ Gas YouTube Condensed Gas Meaning Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. Condensate gases are the result of the transition of a gaseous substance into a liquid state due to a drop in pressure or temperature. Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+. Condensed Gas Meaning.
From sciencenotes.org
Noble Gas Configuration Shorthand Electron Configuration Condensed Gas Meaning During condensation, atoms and molecules form clusters. Condensate gases are the result of the transition of a gaseous substance into a liquid state due to a drop in pressure or temperature. Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that can condense from gas when the. Solids and liquids have particles that are fairly. Condensed Gas Meaning.
From gamesmartz.com
Gas Definition & Image GameSmartz Condensed Gas Meaning During condensation, atoms and molecules form clusters. Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that can condense from gas when the. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. At temperatures below 31°c (the critical temperature), co 2. Condensed Gas Meaning.
From www.pinterest.co.uk
Phase Change Diagram and Definition Change, Condensed matter physics Condensed Gas Meaning Eventually the clusters reach sufficient mass to form droplets. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that can condense from gas when the. During condensation, atoms and molecules form clusters. It. Condensed Gas Meaning.
From www.slideserve.com
PPT STATES OF MATTER PowerPoint Presentation, free download ID595293 Condensed Gas Meaning Eventually the clusters reach sufficient mass to form droplets. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). Condensation is the change in the state of matter from the gas phase to the liquid phase. During condensation, atoms and molecules form clusters. Solids and liquids have particles. Condensed Gas Meaning.
From www.chegg.com
Solved Write the condensed (noblegas) electron Condensed Gas Meaning For example, within a cloud, water nucleates around a dust, pollen, or microbial particle. Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that can condense from gas when the. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. At temperatures. Condensed Gas Meaning.
From anyquotedistrictjibril.blogspot.com
The Best 9 What Is Condensation In Science anyquotedistrictjibril Condensed Gas Meaning Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that can condense from gas when the. It is the reverse of vaporization. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. For example, within a cloud, water nucleates around a dust,. Condensed Gas Meaning.
From www.researchgate.net
Examples of codes, condensed meaning units, subthemes and themes Condensed Gas Meaning For example, within a cloud, water nucleates around a dust, pollen, or microbial particle. Eventually the clusters reach sufficient mass to form droplets. Condensate gases are the result of the transition of a gaseous substance into a liquid state due to a drop in pressure or temperature. Condensation is the change in the state of matter from the gas phase. Condensed Gas Meaning.
From www.slideserve.com
PPT Introduction to the Gas Laws PowerPoint Presentation, free Condensed Gas Meaning For example, within a cloud, water nucleates around a dust, pollen, or microbial particle. Condensate gases are the result of the transition of a gaseous substance into a liquid state due to a drop in pressure or temperature. Condensation is the change in the state of matter from the gas phase to the liquid phase. Eventually the clusters reach sufficient. Condensed Gas Meaning.
From mungfali.com
Solids Liquids Gases Chart Condensed Gas Meaning Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that can condense from gas when the. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even. Condensed Gas Meaning.
From www.slideserve.com
PPT Water States of Matter PowerPoint Presentation, free download Condensed Gas Meaning It is the reverse of vaporization. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). Condensation is the change in the state of matter from the gas phase to the liquid phase. Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that. Condensed Gas Meaning.
From www.slideserve.com
PPT Chemistry 100 PowerPoint Presentation, free download ID2464549 Condensed Gas Meaning During condensation, atoms and molecules form clusters. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). Condensation is the change in the state of. Condensed Gas Meaning.
From www.slideserve.com
PPT GasCondensed Phase Interactions PowerPoint Presentation, free Condensed Gas Meaning Condensation is the change in the state of matter from the gas phase to the liquid phase. Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that can condense from gas when the. For example, within a cloud, water nucleates around a dust, pollen, or microbial particle. During condensation, atoms and molecules form clusters. It. Condensed Gas Meaning.
From eschool.iaspaper.net
What is a Gas Introduction & Modify Eschool Condensed Gas Meaning For example, within a cloud, water nucleates around a dust, pollen, or microbial particle. It is the reverse of vaporization. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at. Condensed Gas Meaning.
From www.slideserve.com
PPT GasCondensed Phase Interactions PowerPoint Presentation, free Condensed Gas Meaning During condensation, atoms and molecules form clusters. Condensation is the change in the state of matter from the gas phase to the liquid phase. Condensate gases are the result of the transition of a gaseous substance into a liquid state due to a drop in pressure or temperature. Solids and liquids have particles that are fairly close to one another,. Condensed Gas Meaning.
From www.britannica.com
phase Definition & Facts Britannica Condensed Gas Meaning Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that can condense from gas when the. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. Condensate gases are the result of the transition of a gaseous substance into a liquid state. Condensed Gas Meaning.
From sciencenotes.org
Examples of Gases What Is a Gas? Condensed Gas Meaning Eventually the clusters reach sufficient mass to form droplets. During condensation, atoms and molecules form clusters. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure. Condensed Gas Meaning.
From www.wikihow.com
How to Write a Noble Gas Configuration for Atoms of an Element Condensed Gas Meaning Condensate gases are the result of the transition of a gaseous substance into a liquid state due to a drop in pressure or temperature. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). Condensation is the change in the state of matter from the gas phase to. Condensed Gas Meaning.
From maggieknoesampson.blogspot.com
Neon Gas Formula MaggieknoeSampson Condensed Gas Meaning During condensation, atoms and molecules form clusters. Eventually the clusters reach sufficient mass to form droplets. Condensation is the change in the state of matter from the gas phase to the liquid phase. It is the reverse of vaporization. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them. Condensed Gas Meaning.
From www.slideserve.com
PPT Unit 6 Gases & The Molecular Theory PowerPoint Condensed Gas Meaning Condensation is the change in the state of matter from the gas phase to the liquid phase. Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that can condense from gas when the. Eventually the clusters reach sufficient mass to form droplets. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an. Condensed Gas Meaning.
From www.slideserve.com
PPT Gases and Gas Laws PowerPoint Presentation, free download ID Condensed Gas Meaning It is the reverse of vaporization. During condensation, atoms and molecules form clusters. Condensation is the change in the state of matter from the gas phase to the liquid phase. Condensate gases are the result of the transition of a gaseous substance into a liquid state due to a drop in pressure or temperature. Gas condensates are liquids, generally straight. Condensed Gas Meaning.
From www.slideserve.com
PPT The Condensed Phase PowerPoint Presentation, free download ID Condensed Gas Meaning Condensate gases are the result of the transition of a gaseous substance into a liquid state due to a drop in pressure or temperature. It is the reverse of vaporization. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). Solids and liquids have particles that are fairly. Condensed Gas Meaning.
From www.britannica.com
Ideal gas law Definition, Formula, & Facts Britannica Condensed Gas Meaning Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). Condensate gases are the result of the transition of a gaseous substance into a liquid. Condensed Gas Meaning.
From www.worksheetsplanet.com
What is a Gas Definition of Gas Condensed Gas Meaning Condensation is the change in the state of matter from the gas phase to the liquid phase. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). Condensate gases are the result of the transition of a gaseous substance into a liquid state due to a drop in. Condensed Gas Meaning.
From topblogtenz.com
Abbreviated electron configuration calculator [Noble gas] Condensed Gas Meaning Condensation is the change in the state of matter from the gas phase to the liquid phase. Eventually the clusters reach sufficient mass to form droplets. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. It is the reverse of vaporization. Condensate gases are the result. Condensed Gas Meaning.
From marketbusinessnews.com
Greenhouse gas (GHG) meaning and several examples Condensed Gas Meaning For example, within a cloud, water nucleates around a dust, pollen, or microbial particle. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). During condensation, atoms and molecules form clusters. Condensation is the change in the state of matter from the gas phase to the liquid phase.. Condensed Gas Meaning.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Condensed Gas Meaning Gas condensates are liquids, generally straight chain alkanes in the c2 to c6+ range, that can condense from gas when the. For example, within a cloud, water nucleates around a dust, pollen, or microbial particle. It is the reverse of vaporization. During condensation, atoms and molecules form clusters. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like. Condensed Gas Meaning.
From study.com
Types of Condensation Lesson Condensed Gas Meaning It is the reverse of vaporization. At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). For example, within a cloud, water nucleates around a dust, pollen, or microbial particle. Condensation is the change in the state of matter from the gas phase to the liquid phase. Eventually. Condensed Gas Meaning.
From www.youtube.com
States of Matter (Phases of Matter) Solids, Liquids, and Gases YouTube Condensed Gas Meaning At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). Condensation is the change in the state of matter from the gas phase to the liquid phase. Eventually the clusters reach sufficient mass to form droplets. It is the reverse of vaporization. Condensate gases are the result of. Condensed Gas Meaning.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation ID1115834 Condensed Gas Meaning At temperatures below 31°c (the critical temperature), co 2 acts somewhat like an ideal gas even at a rather high pressure (). It is the reverse of vaporization. During condensation, atoms and molecules form clusters. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. Condensation is. Condensed Gas Meaning.