Instrument Verification Procedure . Sampling of assay validation and. Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Find out more about the different test methods for measuring instruments in the wika blog. Instrument validation is a series of processes performed according to a specified procedure to verify an instrument meets the expected quality standards. These known measurements are called. What is the difference between a verification and a calibration? Studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for external. Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. Policies and procedures for the introduction of new tests, methods, or instruments. Verification is typically a qualitative provision of objective evidence that a given measurement fulfills specified requirements. Initial verification seeks to ensure that measuring instruments that are to be put into service conform to an approved pattern and to.
from ciqa.net
Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Instrument validation is a series of processes performed according to a specified procedure to verify an instrument meets the expected quality standards. Sampling of assay validation and. Verification is typically a qualitative provision of objective evidence that a given measurement fulfills specified requirements. These known measurements are called. Initial verification seeks to ensure that measuring instruments that are to be put into service conform to an approved pattern and to. Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. What is the difference between a verification and a calibration? Studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for external. Find out more about the different test methods for measuring instruments in the wika blog.
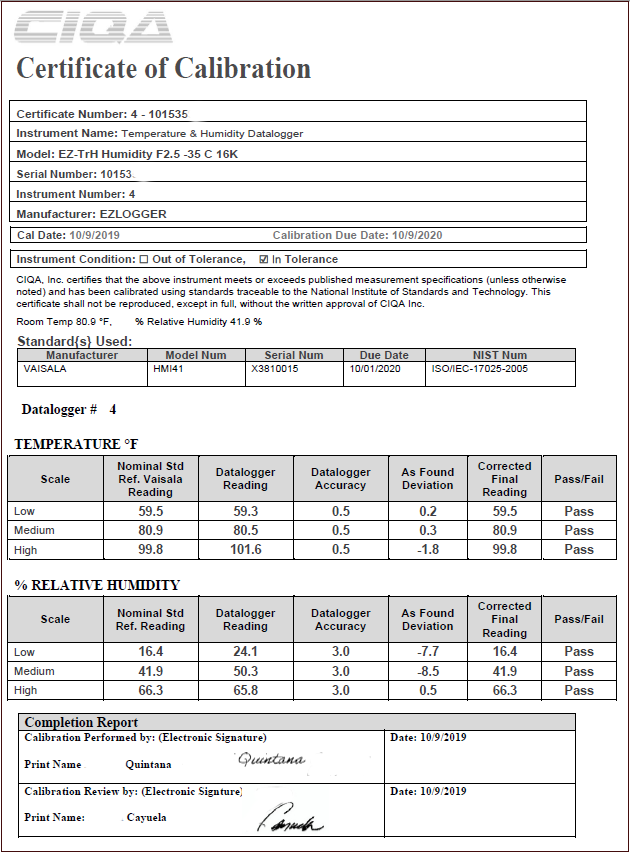
What is a Calibration Certificate or Calibration Record as per ISO17025?
Instrument Verification Procedure Find out more about the different test methods for measuring instruments in the wika blog. Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. Sampling of assay validation and. Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Initial verification seeks to ensure that measuring instruments that are to be put into service conform to an approved pattern and to. Studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for external. Find out more about the different test methods for measuring instruments in the wika blog. These known measurements are called. Policies and procedures for the introduction of new tests, methods, or instruments. Instrument validation is a series of processes performed according to a specified procedure to verify an instrument meets the expected quality standards. What is the difference between a verification and a calibration? Verification is typically a qualitative provision of objective evidence that a given measurement fulfills specified requirements.
From www.researchgate.net
Flowchart of the verification procedure. Download Scientific Diagram Instrument Verification Procedure Initial verification seeks to ensure that measuring instruments that are to be put into service conform to an approved pattern and to. Studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for external. Verification is typically a qualitative provision of objective evidence that a given measurement fulfills specified requirements. Calibration is a procedure. Instrument Verification Procedure.
From cmsmedtech.com
Free ISO 13485 Process Validation Template Instrument Verification Procedure Policies and procedures for the introduction of new tests, methods, or instruments. What is the difference between a verification and a calibration? Verification is typically a qualitative provision of objective evidence that a given measurement fulfills specified requirements. Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. Find out more about the different. Instrument Verification Procedure.
From issuu.com
IOPQ Freezer Validation Template Sample by Pharmi Med Ltd Issuu Instrument Verification Procedure Sampling of assay validation and. Verification is typically a qualitative provision of objective evidence that a given measurement fulfills specified requirements. Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. What is the difference between a verification and a calibration? Studies that demonstrate the performance characteristics of the instrument or assay must be. Instrument Verification Procedure.
From pdfslide.net
(PDF) Verification Procedure for Accuracy and Precision Sipoch Instrument Verification Procedure Policies and procedures for the introduction of new tests, methods, or instruments. Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. What is the difference between a verification and a calibration? Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. Initial verification seeks to ensure. Instrument Verification Procedure.
From ciqa.net
How to create a Validation Master Plan in 5 steps. Templates & more Instrument Verification Procedure What is the difference between a verification and a calibration? Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Initial verification seeks to ensure that measuring instruments that are to be put into service conform to an approved pattern and to. Sampling of assay validation and. Calibration is a procedure for establishing. Instrument Verification Procedure.
From vru.vibrationresearch.com
Calibration Verification Checklist VRU Instrument Verification Procedure What is the difference between a verification and a calibration? Instrument validation is a series of processes performed according to a specified procedure to verify an instrument meets the expected quality standards. Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Policies and procedures for the introduction of new tests, methods, or. Instrument Verification Procedure.
From www.scribd.com
Instrument Validation and Inspection Methods PDF Verification And Instrument Verification Procedure Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Find out more about the different test methods for measuring instruments in the wika blog. Instrument validation is a series of processes performed according to a specified procedure to verify an instrument meets the expected quality standards. Policies and procedures for the introduction. Instrument Verification Procedure.
From greenpegacademy.com
Instrument Verification, Maintenance & Calibration GACA Instrument Verification Procedure Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Find out more about the different test methods for measuring instruments in the wika blog. Policies and procedures for the introduction of new tests, methods, or instruments. Verification is typically a qualitative provision of objective evidence that a given measurement fulfills specified requirements.. Instrument Verification Procedure.
From www.slideserve.com
PPT THEMIS Instrument Test Review Instrument Verification Program Instrument Verification Procedure Studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for external. Sampling of assay validation and. Policies and procedures for the introduction of new tests, methods, or instruments. Initial verification seeks to ensure that measuring instruments that are to be put into service conform to an approved pattern and to. Find out more. Instrument Verification Procedure.
From www.scribd.com
7 Calibration Instrumentation Calibration Instrument Verification Procedure Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Instrument validation is a series of processes performed according to a specified procedure to verify an instrument meets the expected quality standards. What is the difference between a verification and a calibration? These known measurements are called. Calibration is a procedure for establishing. Instrument Verification Procedure.
From www.scribd.com
Calibration Procedure Instrument Verification Procedure Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Sampling of assay validation and. Find out more about the different test methods for measuring instruments in the wika blog. Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. Studies that demonstrate the performance characteristics of. Instrument Verification Procedure.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Instrument Verification Procedure Find out more about the different test methods for measuring instruments in the wika blog. Verification is typically a qualitative provision of objective evidence that a given measurement fulfills specified requirements. Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Studies that demonstrate the performance characteristics of the instrument or assay must. Instrument Verification Procedure.
From www.researchgate.net
List of instruments used for this verification process. Download Instrument Verification Procedure These known measurements are called. Instrument validation is a series of processes performed according to a specified procedure to verify an instrument meets the expected quality standards. Policies and procedures for the introduction of new tests, methods, or instruments. Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. Sampling of assay validation and.. Instrument Verification Procedure.
From guideline-sop.com
Process Validation (PV) & Verification of Drug Product Guidelines SOPs Instrument Verification Procedure Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Instrument validation is a series of processes performed according to a specified procedure to verify an instrument meets the expected quality standards. Find out more about the different test methods for measuring instruments in the wika blog. Initial verification seeks to ensure that. Instrument Verification Procedure.
From fabalabse.com
What are the two types of verification? Leia aqui What are the 2 Instrument Verification Procedure Policies and procedures for the introduction of new tests, methods, or instruments. Sampling of assay validation and. Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. Instrument validation is a series of processes performed according to. Instrument Verification Procedure.
From ekdoseispelasgos.blogspot.com
Equipment Validation Template Master Template Instrument Verification Procedure These known measurements are called. What is the difference between a verification and a calibration? Instrument validation is a series of processes performed according to a specified procedure to verify an instrument meets the expected quality standards. Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Find out more about the different. Instrument Verification Procedure.
From ciqa.net
What is a Calibration Certificate or Calibration Record as per ISO17025? Instrument Verification Procedure Sampling of assay validation and. Verification is typically a qualitative provision of objective evidence that a given measurement fulfills specified requirements. Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. These known measurements are called. Find out more about the different test methods for measuring instruments in the wika blog. Studies that demonstrate. Instrument Verification Procedure.
From calibrationawareness.com
How to Differentiate Calibration, Verification, and Validation Instrument Verification Procedure Initial verification seeks to ensure that measuring instruments that are to be put into service conform to an approved pattern and to. Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Verification is typically a qualitative. Instrument Verification Procedure.
From instrumentationtools.com
SIS Verification & Validation Safety Instrumented System Instrument Verification Procedure Initial verification seeks to ensure that measuring instruments that are to be put into service conform to an approved pattern and to. Sampling of assay validation and. Find out more about the different test methods for measuring instruments in the wika blog. Studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for external.. Instrument Verification Procedure.
From www.presentationeze.com
Equipment Validation Facility Qualification Material Instrument Verification Procedure Find out more about the different test methods for measuring instruments in the wika blog. Verification is typically a qualitative provision of objective evidence that a given measurement fulfills specified requirements. Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. Policies and procedures for the introduction of new tests, methods, or instruments. Studies. Instrument Verification Procedure.
From www.youtube.com
Calibration of measuring instruments Definition of calibration Instrument Verification Procedure Find out more about the different test methods for measuring instruments in the wika blog. What is the difference between a verification and a calibration? Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. Policies and procedures for the introduction of new tests, methods, or instruments. Studies that demonstrate the performance characteristics of. Instrument Verification Procedure.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Instrument Verification Procedure Sampling of assay validation and. These known measurements are called. What is the difference between a verification and a calibration? Find out more about the different test methods for measuring instruments in the wika blog. Policies and procedures for the introduction of new tests, methods, or instruments. Calibration is a procedure for establishing a relationship between a quantitative measurement and. Instrument Verification Procedure.
From www.studocu.com
Instrument Installation Verification Instrument Installation Instrument Verification Procedure Verification is typically a qualitative provision of objective evidence that a given measurement fulfills specified requirements. Studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for external. Instrument validation is a series of processes performed according to a specified procedure to verify an instrument meets the expected quality standards. Policies and procedures for. Instrument Verification Procedure.
From www.getreskilled.com
Equipment Validation Protocol Step by Step Writing Guide Instrument Verification Procedure Instrument validation is a series of processes performed according to a specified procedure to verify an instrument meets the expected quality standards. Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. Find out more about the different test methods for measuring instruments in the wika blog. These known measurements are called. Calibration refers. Instrument Verification Procedure.
From instrumentationtools.com
How to Create Calibration Records? Instrumentation and Control Instrument Verification Procedure Find out more about the different test methods for measuring instruments in the wika blog. Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Verification is typically a qualitative provision of objective evidence that a given measurement fulfills specified requirements. Studies that demonstrate the performance characteristics of the instrument or assay must. Instrument Verification Procedure.
From www.researchgate.net
Steps after the process of instrument validation. Download Scientific Instrument Verification Procedure Find out more about the different test methods for measuring instruments in the wika blog. Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. Verification is typically a qualitative provision of objective evidence that a given measurement fulfills specified requirements. Studies that demonstrate the performance characteristics of the instrument or assay must be. Instrument Verification Procedure.
From www.scribd.com
Calibration Procedure PDF Calibration Engineering Instrument Verification Procedure Sampling of assay validation and. These known measurements are called. Policies and procedures for the introduction of new tests, methods, or instruments. Initial verification seeks to ensure that measuring instruments that are to be put into service conform to an approved pattern and to. What is the difference between a verification and a calibration? Calibration refers to the process of. Instrument Verification Procedure.
From labomat.eu
Thickness gauge Calibration, Verification and Adjustment Labomat Instrument Verification Procedure Policies and procedures for the introduction of new tests, methods, or instruments. Initial verification seeks to ensure that measuring instruments that are to be put into service conform to an approved pattern and to. Find out more about the different test methods for measuring instruments in the wika blog. Studies that demonstrate the performance characteristics of the instrument or assay. Instrument Verification Procedure.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Instrument Verification Procedure Instrument validation is a series of processes performed according to a specified procedure to verify an instrument meets the expected quality standards. What is the difference between a verification and a calibration? Policies and procedures for the introduction of new tests, methods, or instruments. Initial verification seeks to ensure that measuring instruments that are to be put into service conform. Instrument Verification Procedure.
From pharmabeginers.com
SOP for Instrument Calibration (Internal & Third Party) Pharma Beginners Instrument Verification Procedure Initial verification seeks to ensure that measuring instruments that are to be put into service conform to an approved pattern and to. Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. Find out more about the different test methods for measuring instruments in the wika blog. Sampling of assay validation and. Calibration refers. Instrument Verification Procedure.
From www.formica.ai
Explained KYC Verification Process and Preventing User Friction Instrument Verification Procedure Calibration is a procedure for establishing a relationship between a quantitative measurement and a known reference. Initial verification seeks to ensure that measuring instruments that are to be put into service conform to an approved pattern and to. These known measurements are called. Studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for. Instrument Verification Procedure.
From www.scsconcept.com
Torque calibration procedures SCS Concept Accredited laboratory SCS Instrument Verification Procedure Instrument validation is a series of processes performed according to a specified procedure to verify an instrument meets the expected quality standards. Calibration refers to the process of comparing the accuracy of an instrument’s measurements to a known standard. Find out more about the different test methods for measuring instruments in the wika blog. Studies that demonstrate the performance characteristics. Instrument Verification Procedure.
From safetyspecialists.com.au
Electrical Inspection and Test Plan Checklist NECA Safety Specialists Instrument Verification Procedure These known measurements are called. Policies and procedures for the introduction of new tests, methods, or instruments. Initial verification seeks to ensure that measuring instruments that are to be put into service conform to an approved pattern and to. Instrument validation is a series of processes performed according to a specified procedure to verify an instrument meets the expected quality. Instrument Verification Procedure.
From www.slideserve.com
PPT THEMIS Instrument Test Review Instrument Verification Program Instrument Verification Procedure Find out more about the different test methods for measuring instruments in the wika blog. Sampling of assay validation and. Policies and procedures for the introduction of new tests, methods, or instruments. Verification is typically a qualitative provision of objective evidence that a given measurement fulfills specified requirements. Instrument validation is a series of processes performed according to a specified. Instrument Verification Procedure.
From hearing-balance.academy
Aurical Start Up Guide Hearing instrument verification using RECD Instrument Verification Procedure Policies and procedures for the introduction of new tests, methods, or instruments. Initial verification seeks to ensure that measuring instruments that are to be put into service conform to an approved pattern and to. Studies that demonstrate the performance characteristics of the instrument or assay must be documented and accessible for external. Calibration is a procedure for establishing a relationship. Instrument Verification Procedure.