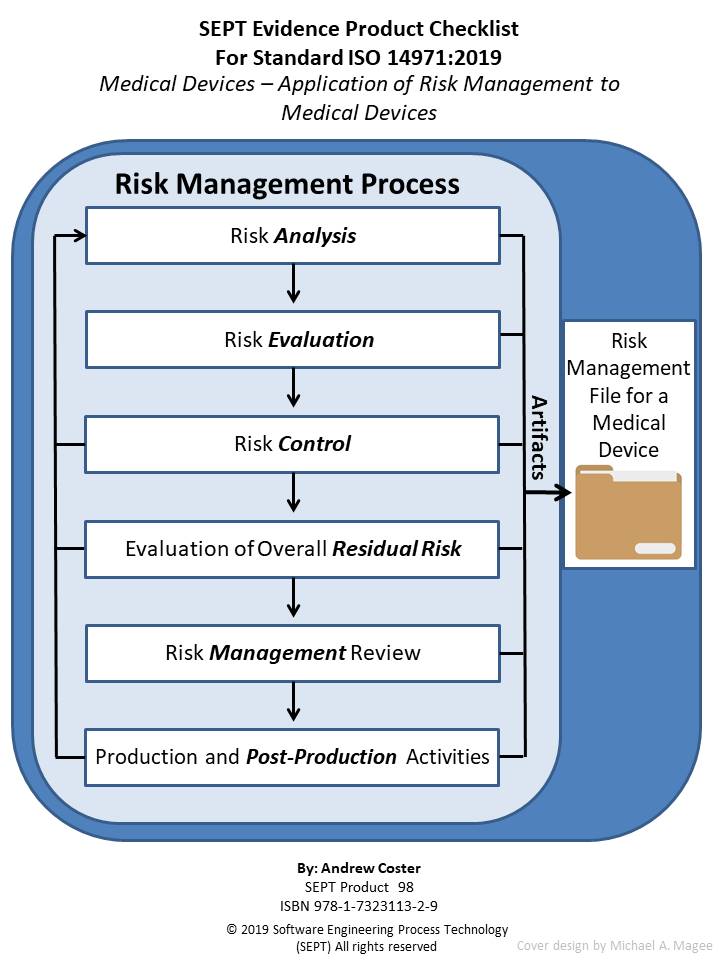
Medical Device Control Plan . Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products.
from mungfali.com
Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products.
Medical Device Risk Management Plan Template
Medical Device Control Plan Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process.
From www.outsourcedpharma.com
Managing Risk For Medical Device Clinical Trials Medical Device Control Plan Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Food. Medical Device Control Plan.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Control Plan Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Explaining quality control processes in the medical device industry. Medical Device Control Plan.
From simbex.com
ISO 14971 Managing Risk in Medical Device Development Simbex Medical Device Control Plan Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food. Medical Device Control Plan.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Control Plan The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Safe medical device act of 1990 authorized. Medical Device Control Plan.
From www.aplyon.com
Medical Device Design and Development Procedures Medical Device Control Plan The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Safe medical device act of 1990 authorized. Medical Device Control Plan.
From www.dotcompliance.com
Quality Management System (QMS) for Medical Device Dot Compliance Medical Device Control Plan Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Safe medical device act of 1990 authorized. Medical Device Control Plan.
From www.springboard.pro
Design controls in medical device development Ensuring safety and Medical Device Control Plan Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food. Medical Device Control Plan.
From starfishmedical.com
5 Top Annual Plan Medical Device Design and Development Process Medical Device Control Plan The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Safe medical device act of 1990 authorized. Medical Device Control Plan.
From template.mapadapalavra.ba.gov.br
Medical Device Design And Development Plan Template Medical Device Control Plan Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food. Medical Device Control Plan.
From mungfali.com
Medical Device Risk Management Plan Template Medical Device Control Plan The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Explaining quality control processes in the medical device industry. Medical Device Control Plan.
From www.aplyon.com
Medical Device Clinical Investigation Plan (CIP) ISO 141552020 Compliant Medical Device Control Plan Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Safe medical device act of 1990 authorized. Medical Device Control Plan.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Control Plan Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Food. Medical Device Control Plan.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Medical Device Control Plan Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Explaining quality control processes in the medical device industry. Medical Device Control Plan.
From medicaldevicehq.com
How to write instructions for use for medical devices Medical Device HQ Medical Device Control Plan Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Explaining quality control processes in the medical device industry. Medical Device Control Plan.
From studylib.net
HRP914 2014 Medical Device Management Plan Medical Device Control Plan Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Food. Medical Device Control Plan.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Control Plan Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food. Medical Device Control Plan.
From www.cognidox.com
Medical Device Development Guide Medical Device Control Plan Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Food. Medical Device Control Plan.
From www.qualitymeddev.com
IEC 62304 Medical Device Software Overview of the Main Requirements Medical Device Control Plan Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Safe medical device act of 1990 authorized. Medical Device Control Plan.
From sunstonepilot.com
What Are Medical Device Design Controls? Sunstone Pilot, Inc. Medical Device Control Plan The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Explaining quality control processes in the medical device industry. Medical Device Control Plan.
From medicaldevicehq.com
The Perfect Project Process Medical Device Product Development Medical Device Control Plan Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. The process fmea and. Medical Device Control Plan.
From www.orielstat.com
Medical Device QMS 101 What It Is, Where It’s Required, and Key Medical Device Control Plan Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. The process fmea and. Medical Device Control Plan.
From www.dotcompliance.com
The Essential Guide to ISO 14971 Dot Compliance Medical Device Control Plan Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. The process fmea and. Medical Device Control Plan.
From www.orielstat.com
Basics of Medical Device Design Controls What, Why, and How Oriel Medical Device Control Plan Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. The process fmea and. Medical Device Control Plan.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Control Plan The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Safe medical device act of 1990 authorized. Medical Device Control Plan.
From www.qmdocs.com
Medical Device Design Control SOP Medical Device Control Plan Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Safe medical device act of 1990 authorized. Medical Device Control Plan.
From www.orielstat.com
Creating a Medical Device Risk Management Plan and Doing Analysis Medical Device Control Plan Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Safe medical device act of 1990 authorized. Medical Device Control Plan.
From issuu.com
md001medicaldevicesdesigncontrolsop1.022 by qmdocs qmdocs Issuu Medical Device Control Plan The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Food. Medical Device Control Plan.
From www.pinterest.com
Image result for design control phases medical device Medical device Medical Device Control Plan Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food. Medical Device Control Plan.
From www.scribd.com
Clcgp004 Medical Devices Governance Management Policy v6 Medical Medical Device Control Plan The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Explaining quality control processes in the medical device industry. Medical Device Control Plan.
From www.qualio.com
Ultimate guide to medical device design controls Medical Device Control Plan The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Food. Medical Device Control Plan.
From www.orielstat.com
Basics of Medical Device Design Controls What, Why, and How Oriel Medical Device Control Plan The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Safe medical device act of 1990 authorized. Medical Device Control Plan.
From www.pannam.com
Ultimate Guide to Medical Device Design and Development Pannam Medical Device Control Plan The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Food. Medical Device Control Plan.
From www.modernrequirements.com
Facilitate the Medical Device Design Controls Modern Requirements Medical Device Control Plan Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Explaining quality control processes in the medical device industry. Medical Device Control Plan.
From old.sermitsiaq.ag
Medical Device Project Plan Template Medical Device Control Plan The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Food and drug administration (fda), health canada, and the u.k.’s medicines and healthcare products. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Explaining quality control processes in the medical device industry. Medical Device Control Plan.
From medicaldevicehq.com
Using MS Excel for medical device risk management Medical Device Control Plan Explaining quality control processes in the medical device industry with actionable steps for companies to use when evaluating their quality control process. The process fmea and its supporting process control plan provide a systematic method for accomplishing this task. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Food. Medical Device Control Plan.