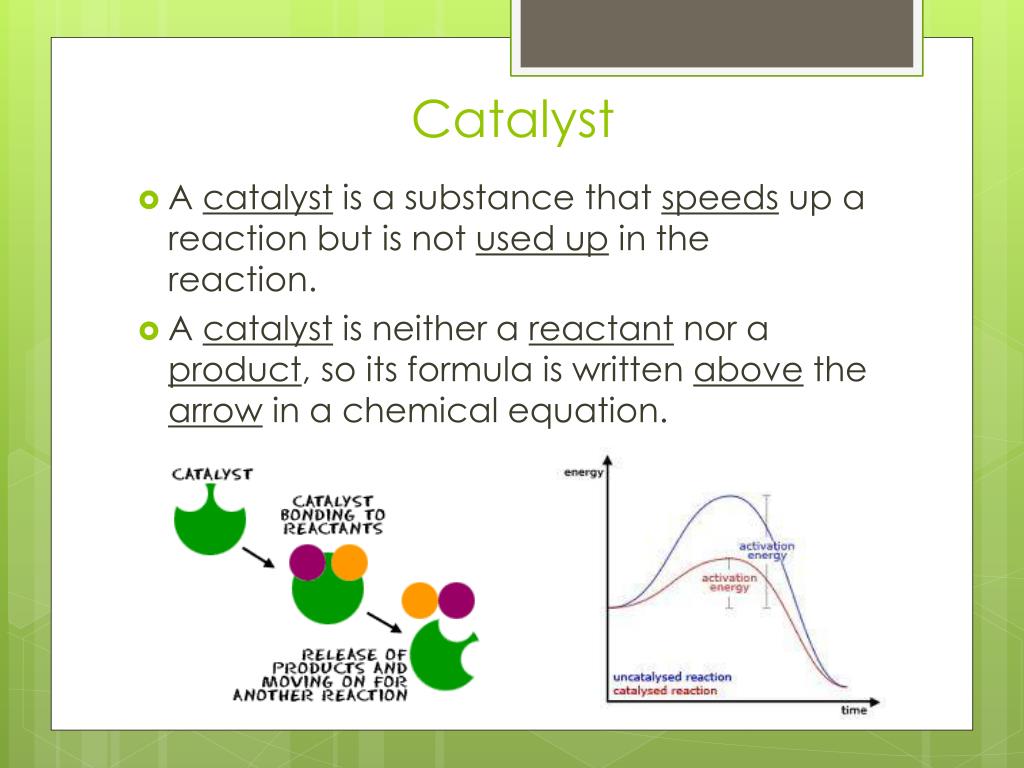
Catalyst Function Chemistry . Catalysts participate in a chemical reaction and increase its rate. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. List examples of catalysis in natural and industrial. They do not appear in the reaction’s net equation and are not consumed during the reaction. List examples of catalysis in natural and industrial processes. Enzymes are naturally occurring catalysts. This process is called catalysis. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts allow a reaction to proceed via a.
from www.slideserve.com
Enzymes are naturally occurring catalysts. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. List examples of catalysis in natural and industrial processes. Catalysts participate in a chemical reaction and increase its rate. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts allow a reaction to proceed via a. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts can be homogenous (in the same phase as the reactants) or.
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free
Catalyst Function Chemistry List examples of catalysis in natural and industrial. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts can be homogenous (in the same phase as the reactants) or. List examples of catalysis in natural and industrial processes. Catalysts participate in a chemical reaction and increase its rate. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation. List examples of catalysis in natural and industrial. Catalysts allow a reaction to proceed via a. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. This process is called catalysis. Enzymes are naturally occurring catalysts. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction.
From www.researchgate.net
The schematic diagram of the catalyst layer. Download Scientific Diagram Catalyst Function Chemistry A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts can be homogenous (in the same phase as the reactants) or. Enzymes are naturally occurring catalysts. Catalysts allow a reaction to proceed via a. They do not appear in the reaction’s net equation and. Catalyst Function Chemistry.
From cekhlmxt.blob.core.windows.net
Catalysts Chemical Process at Edna Vincent blog Catalyst Function Chemistry Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts participate in a chemical reaction and increase its rate. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. They. Catalyst Function Chemistry.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Catalyst Function Chemistry Catalysts allow a reaction to proceed via a. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of. Catalyst Function Chemistry.
From www.slideserve.com
PPT Chapter 14 Chemical Equilibrium PowerPoint Presentation, free Catalyst Function Chemistry Catalysts participate in a chemical reaction and increase its rate. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. List examples of catalysis in natural and industrial processes. Catalysts can be homogenous (in. Catalyst Function Chemistry.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalyst Function Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Explain. Catalyst Function Chemistry.
From exydadzyq.blob.core.windows.net
Catalyst Graph Chemistry at Margaret Wadlington blog Catalyst Function Chemistry List examples of catalysis in natural and industrial. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts participate in a. Catalyst Function Chemistry.
From www.slideserve.com
PPT Transportation PowerPoint Presentation, free download ID969043 Catalyst Function Chemistry Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. List examples of catalysis in natural and industrial processes. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. List examples of catalysis in natural and industrial. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysis,. Catalyst Function Chemistry.
From www.slideserve.com
PPT Enzymes Biological Catalysts PowerPoint Presentation, free Catalyst Function Chemistry Catalysts participate in a chemical reaction and increase its rate. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. List examples of catalysis in natural and industrial. Explain the function. Catalyst Function Chemistry.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Function Chemistry Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. List examples of catalysis in natural and industrial processes. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. They do not. Catalyst Function Chemistry.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Function Chemistry Catalysts allow a reaction to proceed via a. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. List examples of catalysis in natural and industrial processes. This process. Catalyst Function Chemistry.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalyst Function Chemistry Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Enzymes are naturally occurring catalysts. List examples of catalysis in natural and industrial processes. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalyst,. Catalyst Function Chemistry.
From courses.lumenlearning.com
Catalysis Chemistry Catalyst Function Chemistry This process is called catalysis. Catalysts participate in a chemical reaction and increase its rate. List examples of catalysis in natural and industrial processes. List examples of catalysis in natural and industrial. Catalysts allow a reaction to proceed via a. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed. Catalyst Function Chemistry.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Function Chemistry Catalysts participate in a chemical reaction and increase its rate. Catalysts can be homogenous (in the same phase as the reactants) or. This process is called catalysis. Catalysts allow a reaction to proceed via a. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst is a chemical substance that affects the rate. Catalyst Function Chemistry.
From joifdozvf.blob.core.windows.net
What Is A Catalyst In Chemistry Example at Herbert Simpson blog Catalyst Function Chemistry Catalysts can be homogenous (in the same phase as the reactants) or. List examples of catalysis in natural and industrial processes. Catalysts allow a reaction to proceed via a. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. List examples of catalysis in natural and industrial. This process is called catalysis. Catalysts affect the. Catalyst Function Chemistry.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Function Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to proceed via a. Catalysts participate in a chemical reaction and increase its rate. List examples of catalysis in natural and industrial. Catalysts can. Catalyst Function Chemistry.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Function Chemistry Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a chemical reaction and increase its rate. List examples of catalysis in natural and industrial. List examples of catalysis in natural and industrial processes. Enzymes are. Catalyst Function Chemistry.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Function Chemistry Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation. Catalysts can be homogenous (in the same phase as the reactants) or. Enzymes are naturally occurring catalysts. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. They do not appear in the reaction’s net equation and. Catalyst Function Chemistry.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Catalyst Function Chemistry List examples of catalysis in natural and industrial. Catalysts participate in a chemical reaction and increase its rate. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysts. Catalyst Function Chemistry.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Catalyst Function Chemistry Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation. List examples of catalysis in natural and industrial. Catalyst, in chemistry, any. Catalyst Function Chemistry.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 Catalyst Function Chemistry Enzymes are naturally occurring catalysts. Catalysts can be homogenous (in the same phase as the reactants) or. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. List examples of catalysis in natural and industrial processes. List examples of catalysis in natural and industrial. Catalysts. Catalyst Function Chemistry.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalyst Function Chemistry List examples of catalysis in natural and industrial processes. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation. Explain the function of a catalyst in terms. Catalyst Function Chemistry.
From www.slideshare.net
Catalyst Catalyst Function Chemistry They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts can be homogenous (in the same phase as the reactants) or. This process is called catalysis. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts function by allowing the reaction to take place. Catalyst Function Chemistry.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Function Chemistry Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts participate in a chemical reaction and increase its rate. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. They do not appear in. Catalyst Function Chemistry.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2684800 Catalyst Function Chemistry Enzymes are naturally occurring catalysts. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts can be homogenous (in the same phase as the reactants) or. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts participate in a chemical reaction and increase its rate. Catalysts. Catalyst Function Chemistry.
From joilemviq.blob.core.windows.net
Catalyst In Reaction Purpose at John Gainey blog Catalyst Function Chemistry They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts allow a reaction to proceed via a. Enzymes are naturally occurring catalysts. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. List examples of. Catalyst Function Chemistry.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of Catalyst Function Chemistry Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Enzymes are naturally occurring catalysts. List examples of catalysis in natural and industrial. List examples of catalysis in natural and industrial processes. They do not appear in. Catalyst Function Chemistry.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalyst Function Chemistry Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts can be homogenous (in the same phase as the reactants) or. List examples of catalysis in natural and industrial. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed.. Catalyst Function Chemistry.
From www.youtube.com
Types of catalysts AP Chemistry Khan Academy YouTube Catalyst Function Chemistry Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. List examples of catalysis in natural and industrial. Enzymes are naturally occurring catalysts. Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. List examples of. Catalyst Function Chemistry.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Function Chemistry Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts allow a reaction to proceed via a. This process is called catalysis. Catalysts can be homogenous (in the same phase as the reactants) or. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. List examples of catalysis in. Catalyst Function Chemistry.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Function Chemistry Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. This process is called catalysis. Catalysts participate in a chemical reaction and increase its rate. Enzymes are naturally occurring. Catalyst Function Chemistry.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Catalyst Function Chemistry Catalysts allow a reaction to proceed via a. List examples of catalysis in natural and industrial processes. Catalysts can be homogenous (in the same phase as the reactants) or. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Enzymes are naturally occurring catalysts. Catalysts function by allowing the reaction to take place through an. Catalyst Function Chemistry.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Catalyst Function Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. They. Catalyst Function Chemistry.
From www.slideshare.net
Catalysis Catalyst Function Chemistry They do not appear in the reaction’s net equation and are not consumed during the reaction. This process is called catalysis. List examples of catalysis in natural and industrial processes. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation. A catalyst is a chemical substance that affects the rate of a. Catalyst Function Chemistry.
From zymvol.com
All you need to know about enzymes Zymvol Catalyst Function Chemistry Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Enzymes are naturally. Catalyst Function Chemistry.
From klamsflsd.blob.core.windows.net
Catalyst Change A Reaction at Rebecca Miller blog Catalyst Function Chemistry Catalysts allow a reaction to proceed via a. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. List examples of catalysis in natural and industrial processes. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst is a chemical substance that affects the rate of a. Catalyst Function Chemistry.