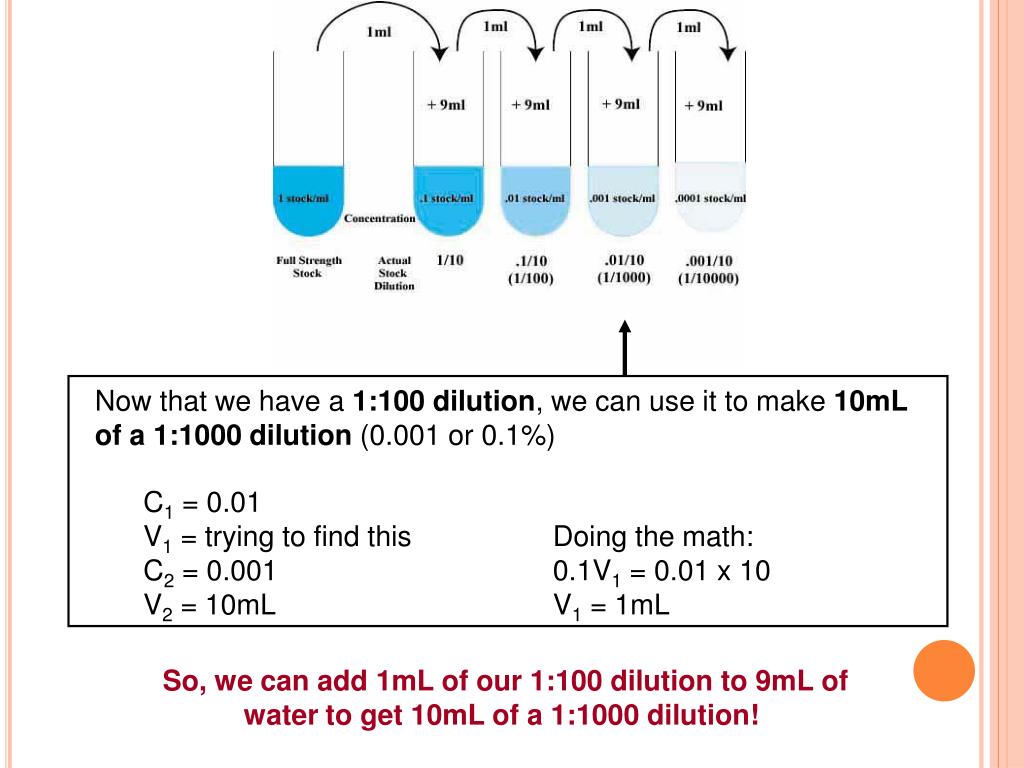
Dilution Problems Meaning . Concentration is the removal of solvent, which increases the concentration of the solute in. We are often concerned with how much solute is dissolved in a given amount of solution. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as water.” diluting a. State whether the concentration of a solution is directly or indirectly proportional to its volume. Explanations and examples of common methods. To dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is thoroughly mixed so as to. These dilution example problems show how to. Understand how stock solutions are used in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration and dilution. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute.
from www.slideserve.com
Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as water.” diluting a. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. We are often concerned with how much solute is dissolved in a given amount of solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. To dilute a solution means to add more solvent without the addition of more solute. Concentration is the removal of solvent, which increases the concentration of the solute in. Explanations and examples of common methods. Of course, the resulting solution is thoroughly mixed so as to. There are many ways of expressing concentration and dilution. Understand how stock solutions are used in the laboratory.
PPT Concentration and Dilution of Solutions PowerPoint Presentation
Dilution Problems Meaning Of course, the resulting solution is thoroughly mixed so as to. Of course, the resulting solution is thoroughly mixed so as to. There are many ways of expressing concentration and dilution. Concentration is the removal of solvent, which increases the concentration of the solute in. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. These dilution example problems show how to. To dilute a solution means to add more solvent without the addition of more solute. We are often concerned with how much solute is dissolved in a given amount of solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as water.” diluting a. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Understand how stock solutions are used in the laboratory. Explanations and examples of common methods.
From www.tffn.net
Solving Dilution Problems A StepbyStep Guide The Enlightened Mindset Dilution Problems Meaning Concentration is the removal of solvent, which increases the concentration of the solute in. There are many ways of expressing concentration and dilution. Understand how stock solutions are used in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Of course, the resulting solution is thoroughly mixed so as to. We. Dilution Problems Meaning.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilution Problems Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. There are many ways of expressing concentration and dilution. To dilute a solution means to add more solvent without the addition. Dilution Problems Meaning.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Problems Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Of course, the resulting solution is thoroughly mixed so as to. We are often concerned with how much solute is dissolved in a given amount of solution. Explanations and examples of common methods. Understand how stock solutions are used in the laboratory. State whether. Dilution Problems Meaning.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID Dilution Problems Meaning To dilute a solution means to add more solvent without the addition of more solute. Concentration is the removal of solvent, which increases the concentration of the solute in. These dilution example problems show how to. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilution is a process where the concentration of. Dilution Problems Meaning.
From www.chegg.com
Solved Practice Dilution Problems Section I 1. How much Dilution Problems Meaning Understand how stock solutions are used in the laboratory. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. We are often concerned with how much solute is dissolved in a given amount of solution. These dilution example problems show how to. Dilution is the process of. Dilution Problems Meaning.
From exynemfqo.blob.core.windows.net
Sample Problems In Dilution at Eddie Edgar blog Dilution Problems Meaning Concentration is the removal of solvent, which increases the concentration of the solute in. We are often concerned with how much solute is dissolved in a given amount of solution. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as water.” diluting a. There are many. Dilution Problems Meaning.
From www.youtube.com
Dilutions Explained with Problems YouTube Dilution Problems Meaning Of course, the resulting solution is thoroughly mixed so as to. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as water.” diluting. Dilution Problems Meaning.
From www.slideserve.com
PPT Solution Problems PowerPoint Presentation, free download ID6135696 Dilution Problems Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration and dilution. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as water.” diluting a. Understand how stock solutions are used in the. Dilution Problems Meaning.
From www.chegg.com
Solved Dilution Problems For problems 711 use the dilution Dilution Problems Meaning Explanations and examples of common methods. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of the solute in. To dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is thoroughly mixed so. Dilution Problems Meaning.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Dilution Problems Meaning To dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is thoroughly mixed so as to. Concentration is the removal of solvent, which increases the concentration of the solute in. These dilution example problems show how to. A dilution is a process where the concentration of a solution is lowered. Dilution Problems Meaning.
From studylib.net
Key to the Practice Dilution Problems Dilution Problems Meaning State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition of more solute. These dilution example problems show how to. Understand how stock solutions are used in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in. Dilution Problems Meaning.
From www.slideserve.com
PPT Chapter 4 Reactions in Aqueous Solutions PowerPoint Presentation Dilution Problems Meaning Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as water.” diluting a. These dilution example problems show how to. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. To dilute a solution means to add more solvent without. Dilution Problems Meaning.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID Dilution Problems Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Of course, the resulting solution is thoroughly mixed so as to. State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition of more solute. Explanations and examples. Dilution Problems Meaning.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Dilution Problems Meaning We are often concerned with how much solute is dissolved in a given amount of solution. To dilute a solution means to add more solvent without the addition of more solute. Explanations and examples of common methods. Understand how stock solutions are used in the laboratory. State whether the concentration of a solution is directly or indirectly proportional to its. Dilution Problems Meaning.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Problems Meaning There are many ways of expressing concentration and dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Of course, the resulting solution is thoroughly mixed so as to. Understand how stock solutions are used in the laboratory.. Dilution Problems Meaning.
From study.com
Solving Applied Dilution Problems Chemistry Dilution Problems Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration and dilution. Understand how stock solutions are used in the laboratory. Of course, the resulting solution is thoroughly mixed so as to. To dilute a solution means to add more solvent without the addition of more solute.. Dilution Problems Meaning.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilution Problems Meaning State whether the concentration of a solution is directly or indirectly proportional to its volume. These dilution example problems show how to. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of the solute in. To dilute a solution means to add more. Dilution Problems Meaning.
From exopkbipv.blob.core.windows.net
Dilution Equation Example Chemistry at Wanda King blog Dilution Problems Meaning Understand how stock solutions are used in the laboratory. Of course, the resulting solution is thoroughly mixed so as to. Concentration is the removal of solvent, which increases the concentration of the solute in. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We are often concerned with how much solute is dissolved. Dilution Problems Meaning.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Problems Meaning State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as water.” diluting a. Concentration is the removal of solvent, which increases the concentration of the solute in. We are often concerned. Dilution Problems Meaning.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Problems Meaning There are many ways of expressing concentration and dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Understand how stock solutions are used in the laboratory. We are often concerned with how much solute is dissolved in a given amount of solution. Concentration is the removal of solvent, which increases the concentration. Dilution Problems Meaning.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID Dilution Problems Meaning Of course, the resulting solution is thoroughly mixed so as to. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. To dilute a solution means to add more solvent without the addition of more solute. Dilution is the process of “lowering the concentration of a solute. Dilution Problems Meaning.
From www.youtube.com
Dilution Practice Problems & Example Problems YouTube Dilution Problems Meaning Concentration is the removal of solvent, which increases the concentration of the solute in. These dilution example problems show how to. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Explanations. Dilution Problems Meaning.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Problems Meaning Of course, the resulting solution is thoroughly mixed so as to. Understand how stock solutions are used in the laboratory. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Concentration is the removal of solvent, which increases the concentration of the solute in. We are often. Dilution Problems Meaning.
From exovgpnzm.blob.core.windows.net
Dilution And Concentration Of Liquid at Nicole Gardner blog Dilution Problems Meaning Explanations and examples of common methods. Concentration is the removal of solvent, which increases the concentration of the solute in. There are many ways of expressing concentration and dilution. These dilution example problems show how to. Understand how stock solutions are used in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the. Dilution Problems Meaning.
From www.youtube.com
Molarity Dilution Practice Problem 2 YouTube Dilution Problems Meaning Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as water.” diluting a. State whether the concentration of a solution is directly or indirectly proportional to its volume. We are often concerned with how much solute is dissolved in a given amount of solution. Explanations and. Dilution Problems Meaning.
From www.youtube.com
GeeklyHub Dilution of Solution Dilution Calculations & Problems Dilution Problems Meaning Concentration is the removal of solvent, which increases the concentration of the solute in. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Of course, the resulting solution is thoroughly mixed so as to. Explanations and examples of. Dilution Problems Meaning.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Problems Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration and dilution. We are often concerned with how much solute is dissolved in a given amount of solution. To dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting. Dilution Problems Meaning.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Problems Meaning A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. These dilution example problems show how to. Concentration is the removal of solvent, which increases the concentration of the solute in. Understand how stock solutions are used in the laboratory. Explanations and examples of common methods. We. Dilution Problems Meaning.
From www.slideserve.com
PPT Microbiology Practical I PowerPoint Presentation, free download Dilution Problems Meaning To dilute a solution means to add more solvent without the addition of more solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration and dilution. Dilution is the process of. Dilution Problems Meaning.
From www.chegg.com
Solved Dilution Problems For problems 7 11 use the dilution Dilution Problems Meaning Of course, the resulting solution is thoroughly mixed so as to. These dilution example problems show how to. Understand how stock solutions are used in the laboratory. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. To dilute a solution means to add more solvent without. Dilution Problems Meaning.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Problems Meaning Explanations and examples of common methods. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. To dilute a solution means to add more solvent without the addition of more solute. Understand how stock solutions are used in the laboratory. Dilution is the process of “lowering the. Dilution Problems Meaning.
From www.youtube.com
DILUTIONDEFINITION AND PRACTICE PROBLEMS YouTube Dilution Problems Meaning Concentration is the removal of solvent, which increases the concentration of the solute in. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. These dilution example problems show how to. We are often concerned with how much solute. Dilution Problems Meaning.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Problems Meaning These dilution example problems show how to. There are many ways of expressing concentration and dilution. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as water.” diluting a. A dilution is a process where the concentration of a solution is lowered by adding solvent to. Dilution Problems Meaning.
From www.slideserve.com
PPT Concentration and Dilution of Solutions PowerPoint Presentation Dilution Problems Meaning These dilution example problems show how to. Understand how stock solutions are used in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as water.” diluting a. There. Dilution Problems Meaning.
From www.youtube.com
How to do Dilution problems? Solved examples YouTube Dilution Problems Meaning Understand how stock solutions are used in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration and dilution. We are often concerned with how much solute is dissolved in a given amount of solution. These dilution example problems show how to. To dilute a. Dilution Problems Meaning.