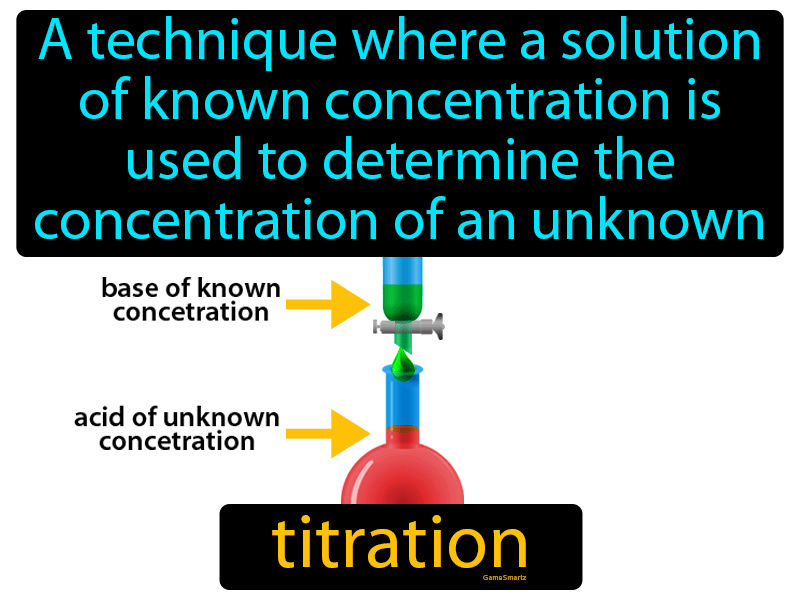
Titration Definition Chemistry Example . Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured.
from gamesmartz.com
Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured.
Titration Definition & Image GameSmartz
Titration Definition Chemistry Example titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis.
From www.thoughtco.com
Redox Titration Definition (Chemistry) Titration Definition Chemistry Example titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of another substance with which. Titration Definition Chemistry Example.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Definition Chemistry Example titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity. Titration Definition Chemistry Example.
From mavink.com
Titration Diagram Labeled Titration Definition Chemistry Example titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of another substance with which the desired constituent reacts in a. Titration Definition Chemistry Example.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration Definition Chemistry Example titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration refers. Titration Definition Chemistry Example.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and Titration Definition Chemistry Example It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. titration is the process in which one solution is added to another solution such. Titration Definition Chemistry Example.
From chem4three.blogspot.ca
CHEMISTRY 11 TITRATIONS Titration Definition Chemistry Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. titration refers to a process where the use of a solution of known concentration. Titration Definition Chemistry Example.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Definition Chemistry Example It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the. Titration Definition Chemistry Example.
From themasterchemistry.com
Iodometric Titration Principle, Example, Advantages Titration Definition Chemistry Example titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in. Titration Definition Chemistry Example.
From tuitiontube.com
Details about AcidBase Titration Lab in Easy Language Tuition Tube Titration Definition Chemistry Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of another substance with. Titration Definition Chemistry Example.
From scienceinfo.com
Titration Definition, 4 Types, Procedure Titration Definition Chemistry Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. It is used in quantitative analytical. Titration Definition Chemistry Example.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Titration Definition Chemistry Example Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. titration is the slow addition of one solution of a known concentration (called. Titration Definition Chemistry Example.
From gamesmartz.com
Titration Definition & Image GameSmartz Titration Definition Chemistry Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to. Titration Definition Chemistry Example.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Definition Chemistry Example It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. Titration Definition Chemistry Example.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Definition Chemistry Example titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. It is used in quantitative analytical. Titration Definition Chemistry Example.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Definition Chemistry Example titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in. Titration Definition Chemistry Example.
From www.slideserve.com
PPT Titrations. . . PowerPoint Presentation, free download ID5607639 Titration Definition Chemistry Example titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. . Titration Definition Chemistry Example.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Definition Chemistry Example titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Titration Definition Chemistry Example.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Definition Chemistry Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration. Titration Definition Chemistry Example.
From printablevozljevrc.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration Definition Chemistry Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of. Titration Definition Chemistry Example.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Definition Chemistry Example titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is. Titration Definition Chemistry Example.
From www.scribd.com
Titration PDF Titration Chemistry Titration Definition Chemistry Example Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Titration Definition Chemistry Example.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Titration Definition Chemistry Example titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is. Titration Definition Chemistry Example.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Definition Chemistry Example titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. titration is the slow addition of one solution of a known concentration (called a. Titration Definition Chemistry Example.
From thechemistrynotes.com
Acidbase Titration 4 Types, Theory, Working Principle Titration Definition Chemistry Example titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity. Titration Definition Chemistry Example.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Titration Definition Chemistry Example titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of another substance with which. Titration Definition Chemistry Example.
From www.slideserve.com
PPT Ch 6 Good Titrations PowerPoint Presentation, free download ID Titration Definition Chemistry Example titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Titration Definition Chemistry Example.
From www.sliderbase.com
Titration Titration Definition Chemistry Example titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of another substance with which the desired constituent reacts in a. Titration Definition Chemistry Example.
From www.scribd.com
titration ( chemistry Experiment report) Titration Analysis Titration Definition Chemistry Example titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the. It is used in quantitative analytical chemistry to determine an unknown concentration of an. Titration Definition Chemistry Example.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Definition Chemistry Example titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration refers to a process where the use of a solution of known concentration. Titration Definition Chemistry Example.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube Titration Definition Chemistry Example titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration. Titration Definition Chemistry Example.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Definition Chemistry Example titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. It is used in quantitative analytical chemistry. Titration Definition Chemistry Example.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Titration Definition Chemistry Example Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. titration refers to a process where the use of a solution of known. Titration Definition Chemistry Example.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Titration Definition Chemistry Example titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. Titration, also known as titrimetry, is a. Titration Definition Chemistry Example.
From www.youtube.com
Titration Definition, steps and formula YouTube Titration Definition Chemistry Example titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. titration, process of chemical analysis in which the quantity of some constituent. Titration Definition Chemistry Example.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Definition Chemistry Example titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the. titration is the process in which one solution is added to another solution. Titration Definition Chemistry Example.