Magnesium Sulfate And Zinc Reaction . zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. In order to determine the relative. magnesium + copper (ii) sulfate → magnesium sulfate + copper. Acid + metal → salt + hydrogen. Lead foil (toxic, dangerous for the environment) magnesium ribbon. Copper, iron, zinc, and magnesium. zinc sulfate (or nitrate (v)) five samples, approximately 1 cm lengths or squares, of each the following metals (note 2): Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. Magnesium + copper sulfate → magnesium sulfate + copper. Mg + cuso₄ → mgso₄ + cu. examine the reactions between various metals and metal salt solutions in this class practical. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. 2(b)(i) to construct a hess’ cycle diagram for the reaction between magnesium and zinc sulfate to form magnesium sulfate and. Mg (s) + h 2 so 4 (aq) mgso 4 (aq). zn + mgso4 = mg + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole.
from mammothmemory.net
In order to determine the relative. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. magnesium displaces both silver and zinc, indicating that it is the most reactive of the three. mgso4 + zn = mg + znso4 is a single displacement (substitution) reaction where one mole of aqueous magnesium sulfate. magnesium + copper (ii) sulfate → magnesium sulfate + copper. 2(b)(i) to construct a hess’ cycle diagram for the reaction between magnesium and zinc sulfate to form magnesium sulfate and. Acid + metal → salt + hydrogen. A metal will displace a less reactive metal from solutions of its compounds. Magnesium + copper (ii) sulfate → magnesium sulfate + copper. we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it displaces other.
More reactive metals displace others in a solution reaction
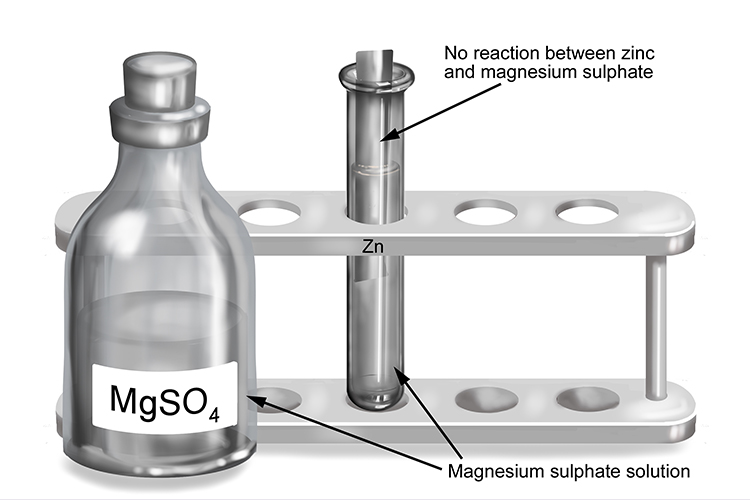
Magnesium Sulfate And Zinc Reaction No reaction is seen if you do things the other way round. Copper, iron, zinc, and magnesium. mgso4 + zn = mg + znso4 is a single displacement (substitution) reaction where one mole of aqueous magnesium sulfate. magnesium displaces both silver and zinc, indicating that it is the most reactive of the three. magnesium reacts with dilute sulfuric acid to give a colourless gas, hydrogen, and a colourless solution of magnesium sulfate. we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it displaces other. a displacement reaction between zinc and lead(ii) nitrate solution. Mg + cuso₄ → mgso₄ + cu. znso4 + mg = zn + mgso4 is a single displacement (substitution) reaction where one mole of aqueous zinc sulfate [znso 4]. Magnesium + copper (ii) sulfate → magnesium sulfate + copper. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. examine the reactions between various metals and metal salt solutions in this class practical. in this experiment we will determine the relative reactivity of 4 metals: Then a zinc electrode is placed in the zinc. Acid + metal → salt + hydrogen. magnesium + copper sulfate → magnesium sulfate + copper.
From mungfali.com
PPT Section 9.1 Naming Ions PowerPoint Presentation Magnesium Sulfate And Zinc Reaction Then a zinc electrode is placed in the zinc. zinc sulfate (or nitrate (v)) five samples, approximately 1 cm lengths or squares, of each the following metals (note 2): 2(b)(i) to construct a hess’ cycle diagram for the reaction between magnesium and zinc sulfate to form magnesium sulfate and. Magnesium + copper sulfate → magnesium sulfate + copper.. Magnesium Sulfate And Zinc Reaction.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Magnesium Sulfate And Zinc Reaction acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. examine the reactions between various metals and metal salt solutions in this class practical. Magnesium + copper sulfate → magnesium sulfate + copper. 2(b)(i) to construct a hess’ cycle diagram for the reaction between magnesium and zinc sulfate to form. Magnesium Sulfate And Zinc Reaction.
From www.youtube.com
How to Write the Net Ionic Equation for Mg + ZnSO4 = Zn + MgSO4 (See Magnesium Sulfate And Zinc Reaction Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. Then a zinc electrode is placed in the zinc. zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. zinc sulfate (or nitrate (v)) five samples, approximately 1 cm lengths. Magnesium Sulfate And Zinc Reaction.
From www.slideserve.com
PPT Magnesium reacts quicker than copper and iron. There is a league Magnesium Sulfate And Zinc Reaction examine the reactions between various metals and metal salt solutions in this class practical. Magnesium + copper sulfate → magnesium sulfate + copper. a zinc sulfate solution is floated on top of the copper sulfate solution; magnesium displaces both silver and zinc, indicating that it is the most reactive of the three. Mg (s) + cuso 4. Magnesium Sulfate And Zinc Reaction.
From inchem.netlify.app
Reaction of zinc powder with cucl2 solution inchem Magnesium Sulfate And Zinc Reaction in this experiment we will determine the relative reactivity of 4 metals: A metal will displace a less reactive metal from solutions of its compounds. No reaction is seen if you do things the other way round. zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and. Magnesium Sulfate And Zinc Reaction.
From www.youtube.com
Magnesium Sulfate + Zinc YouTube Magnesium Sulfate And Zinc Reaction Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. Lead foil (toxic, dangerous for the environment) magnesium ribbon. magnesium displaces both silver and zinc, indicating that it is the most reactive of the three. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. we can examine. Magnesium Sulfate And Zinc Reaction.
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube Magnesium Sulfate And Zinc Reaction magnesium + copper sulfate → magnesium sulfate + copper. Mg (s) + h 2 so 4 (aq) mgso 4 (aq). we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it displaces other. magnesium displaces both silver and zinc, indicating that it is the most reactive of the three. In. Magnesium Sulfate And Zinc Reaction.
From dxonititd.blob.core.windows.net
Magnesium And Zinc Chloride Reaction at Barbara Cleveland blog Magnesium Sulfate And Zinc Reaction Then a zinc electrode is placed in the zinc. mgso4 + zn = mg + znso4 is a single displacement (substitution) reaction where one mole of aqueous magnesium sulfate. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. Lead foil (toxic, dangerous for the environment) magnesium ribbon. Copper, iron, zinc, and magnesium. In. Magnesium Sulfate And Zinc Reaction.
From studylib.net
Displacement Reaction between Magnesium and Copper Sulfate Magnesium Sulfate And Zinc Reaction we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it displaces other. Copper, iron, zinc, and magnesium. in this experiment we will determine the relative reactivity of 4 metals: a zinc sulfate solution is floated on top of the copper sulfate solution; zinc sulfate (or nitrate (v)) five. Magnesium Sulfate And Zinc Reaction.
From edu-rsc-org-s.webvpn.bjmu.doc110.com
Exothermic metal displacement reactions Experiment RSC Education Magnesium Sulfate And Zinc Reaction Lead foil (toxic, dangerous for the environment) magnesium ribbon. zn + mgso4 = mg + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it displaces other. Acid + metal → salt +. Magnesium Sulfate And Zinc Reaction.
From flatworldknowledge.lardbucket.org
Describing Electrochemical Cells Magnesium Sulfate And Zinc Reaction in this experiment we will determine the relative reactivity of 4 metals: Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. No reaction is seen if you do things the other way round. Then a zinc electrode is placed in the zinc. znso4 + mg = zn + mgso4 is a single. Magnesium Sulfate And Zinc Reaction.
From www.nagwa.com
Vidéo question Identifier l’équation qui décrit ce qui se produit au Magnesium Sulfate And Zinc Reaction zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. Lead foil (toxic, dangerous for the environment) magnesium ribbon. magnesium reacts with dilute sulfuric acid to give a colourless gas, hydrogen, and a colourless solution of magnesium sulfate. mgso4 + zn = mg +. Magnesium Sulfate And Zinc Reaction.
From www.youtube.com
WHEN ZINC NITRATE REACTS WITH MAGNESIUM RIBBON YouTube Magnesium Sulfate And Zinc Reaction a zinc sulfate solution is floated on top of the copper sulfate solution; we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it displaces other. A metal will displace a less reactive metal from solutions of its compounds. in this experiment we will determine the relative reactivity of 4. Magnesium Sulfate And Zinc Reaction.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation ID2276630 Magnesium Sulfate And Zinc Reaction Lead foil (toxic, dangerous for the environment) magnesium ribbon. magnesium reacts with dilute sulfuric acid to give a colourless gas, hydrogen, and a colourless solution of magnesium sulfate. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Mg (s) + h 2 so 4 (aq) mgso 4 (aq). zn. Magnesium Sulfate And Zinc Reaction.
From www.youtube.com
Reaction of Zinc in Magnesium and Copper Nitrate YouTube Magnesium Sulfate And Zinc Reaction we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it displaces other. Lead foil (toxic, dangerous for the environment) magnesium ribbon. znso4 + mg = zn + mgso4 is a single displacement (substitution) reaction where one mole of aqueous zinc sulfate [znso 4]. Mg (s) + cuso 4 (aq) →. Magnesium Sulfate And Zinc Reaction.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0146 Science Magnesium Sulfate And Zinc Reaction examine the reactions between various metals and metal salt solutions in this class practical. Mg + cuso₄ → mgso₄ + cu. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and. Magnesium Sulfate And Zinc Reaction.
From www.numerade.com
SOLVEDZinc and magnesium metal each reacts with hydrochloric acid to Magnesium Sulfate And Zinc Reaction zn + mgso4 = mg + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. magnesium displaces both silver and zinc, indicating that it is the most reactive of the three. Then a zinc electrode is placed in the zinc. The experiment reinforces ideas about energy changes during reactions, the. Magnesium Sulfate And Zinc Reaction.
From www.youtube.com
How to Balance H2SO4 + Zn = ZnSO4 + H2 (Sulfuric acid + Zinc) YouTube Magnesium Sulfate And Zinc Reaction examine the reactions between various metals and metal salt solutions in this class practical. magnesium reacts with dilute sulfuric acid to give a colourless gas, hydrogen, and a colourless solution of magnesium sulfate. magnesium + copper sulfate → magnesium sulfate + copper. 2(b)(i) to construct a hess’ cycle diagram for the reaction between magnesium and zinc. Magnesium Sulfate And Zinc Reaction.
From www.youtube.com
How to Write the Formula for Magnesium sulfate YouTube Magnesium Sulfate And Zinc Reaction mgso4 + zn = mg + znso4 is a single displacement (substitution) reaction where one mole of aqueous magnesium sulfate. zinc sulfate (or nitrate (v)) five samples, approximately 1 cm lengths or squares, of each the following metals (note 2): znso4 + mg = zn + mgso4 is a single displacement (substitution) reaction where one mole of. Magnesium Sulfate And Zinc Reaction.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Magnesium Sulfate And Zinc Reaction znso4 + mg = zn + mgso4 is a single displacement (substitution) reaction where one mole of aqueous zinc sulfate [znso 4]. Mg + cuso₄ → mgso₄ + cu. mgso4 + zn = mg + znso4 is a single displacement (substitution) reaction where one mole of aqueous magnesium sulfate. zn + mgso4 = mg + zn(so4) is. Magnesium Sulfate And Zinc Reaction.
From dxolqhrho.blob.core.windows.net
What Happens When A Base Reacts With A Base at Larry Childers blog Magnesium Sulfate And Zinc Reaction The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Lead foil (toxic, dangerous for the environment) magnesium ribbon. magnesium reacts with dilute sulfuric acid to give a colourless gas, hydrogen, and a colourless solution of magnesium sulfate. A metal will displace a less reactive metal from solutions. Magnesium Sulfate And Zinc Reaction.
From exowmpjlt.blob.core.windows.net
Zinc Sulfate + Magnesium Equation at Marcia Balser blog Magnesium Sulfate And Zinc Reaction magnesium + copper (ii) sulfate → magnesium sulfate + copper. In order to determine the relative. Magnesium + copper sulfate → magnesium sulfate + copper. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. A metal will displace a less reactive metal from solutions of its compounds. magnesium displaces. Magnesium Sulfate And Zinc Reaction.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Magnesium Sulfate And Zinc Reaction Then a zinc electrode is placed in the zinc. zinc sulfate (or nitrate (v)) five samples, approximately 1 cm lengths or squares, of each the following metals (note 2): acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu. Magnesium Sulfate And Zinc Reaction.
From www.youtube.com
Net Ionic Equation for MgSO4 + BaCl2 (Magnesium sulfate and Barium Magnesium Sulfate And Zinc Reaction magnesium displaces both silver and zinc, indicating that it is the most reactive of the three. Copper, iron, zinc, and magnesium. in this experiment we will determine the relative reactivity of 4 metals: Magnesium + copper sulfate → magnesium sulfate + copper. Lead foil (toxic, dangerous for the environment) magnesium ribbon. No reaction is seen if you do. Magnesium Sulfate And Zinc Reaction.
From www.researchgate.net
Schematic diagram of the reactions on the surface of the magnesium Magnesium Sulfate And Zinc Reaction znso4 + mg = zn + mgso4 is a single displacement (substitution) reaction where one mole of aqueous zinc sulfate [znso 4]. magnesium + copper sulfate → magnesium sulfate + copper. in this experiment we will determine the relative reactivity of 4 metals: Zinc is above lead in the reactivity series and you would expect. In order. Magnesium Sulfate And Zinc Reaction.
From exoypnorc.blob.core.windows.net
Metal In Water Reaction at Glenn King blog Magnesium Sulfate And Zinc Reaction Zinc is above lead in the reactivity series and you would expect. mgso4 + zn = mg + znso4 is a single displacement (substitution) reaction where one mole of aqueous magnesium sulfate. a zinc sulfate solution is floated on top of the copper sulfate solution; No reaction is seen if you do things the other way round. . Magnesium Sulfate And Zinc Reaction.
From exotnloud.blob.core.windows.net
Electrochemical Activities Of Graphene at Ruby Flores blog Magnesium Sulfate And Zinc Reaction in this experiment we will determine the relative reactivity of 4 metals: Zinc is above lead in the reactivity series and you would expect. a displacement reaction between zinc and lead(ii) nitrate solution. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. Copper, iron, zinc, and magnesium. Then a zinc electrode is. Magnesium Sulfate And Zinc Reaction.
From mammothmemory.net
More reactive metals displace others in a solution reaction Magnesium Sulfate And Zinc Reaction znso4 + mg = zn + mgso4 is a single displacement (substitution) reaction where one mole of aqueous zinc sulfate [znso 4]. examine the reactions between various metals and metal salt solutions in this class practical. magnesium + copper sulfate → magnesium sulfate + copper. magnesium displaces both silver and zinc, indicating that it is the. Magnesium Sulfate And Zinc Reaction.
From www.youtube.com
Zinc Nitrate + Magnesium YouTube Magnesium Sulfate And Zinc Reaction The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. In order to determine the relative. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. Then a zinc electrode is placed in the zinc. in this experiment we will determine the relative. Magnesium Sulfate And Zinc Reaction.
From schematicmemberfdiche.z22.web.core.windows.net
Magnesium And Lead Reaction Magnesium Sulfate And Zinc Reaction magnesium displaces both silver and zinc, indicating that it is the most reactive of the three. Mg (s) + h 2 so 4 (aq) mgso 4 (aq). Copper, iron, zinc, and magnesium. Then a zinc electrode is placed in the zinc. In order to determine the relative. magnesium + copper sulfate → magnesium sulfate + copper. we. Magnesium Sulfate And Zinc Reaction.
From www.nagwa.com
Question Video Identifying the Equation That Describes What Happens to Magnesium Sulfate And Zinc Reaction A metal will displace a less reactive metal from solutions of its compounds. zn + mgso4 = mg + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. Mg (s) + h 2 so 4 (aq) mgso 4 (aq). Zinc is above lead in the reactivity series and you would expect.. Magnesium Sulfate And Zinc Reaction.
From www.alamy.com
reactivity of different metals with hydrochloric acid calcium magnesium Magnesium Sulfate And Zinc Reaction Then a zinc electrode is placed in the zinc. zn + mgso4 = mg + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. Copper, iron, zinc, and magnesium. acids will react with reactive metals,. Magnesium Sulfate And Zinc Reaction.
From www.youtube.com
Chemical Reaction Experiment magnesium sulfate with sodium carbonate Magnesium Sulfate And Zinc Reaction Zinc is above lead in the reactivity series and you would expect. Then a zinc electrode is placed in the zinc. Acid + metal → salt + hydrogen. In order to determine the relative. magnesium reacts with dilute sulfuric acid to give a colourless gas, hydrogen, and a colourless solution of magnesium sulfate. magnesium + copper sulfate →. Magnesium Sulfate And Zinc Reaction.
From dxomzyoyw.blob.core.windows.net
Zinc Nitrate And Magnesium Reaction at Amanda Fulton blog Magnesium Sulfate And Zinc Reaction zn + mgso4 = mg + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. Magnesium + copper (ii) sulfate → magnesium sulfate + copper. zinc sulfate (or nitrate (v)) five samples, approximately 1 cm lengths or squares, of each the following metals (note 2): Mg (s) + cuso 4. Magnesium Sulfate And Zinc Reaction.
From www.toppr.com
The equation shows the reaction between magnesium and sulphuric acid Magnesium Sulfate And Zinc Reaction In order to determine the relative. Lead foil (toxic, dangerous for the environment) magnesium ribbon. zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. The experiment reinforces ideas about energy. Magnesium Sulfate And Zinc Reaction.