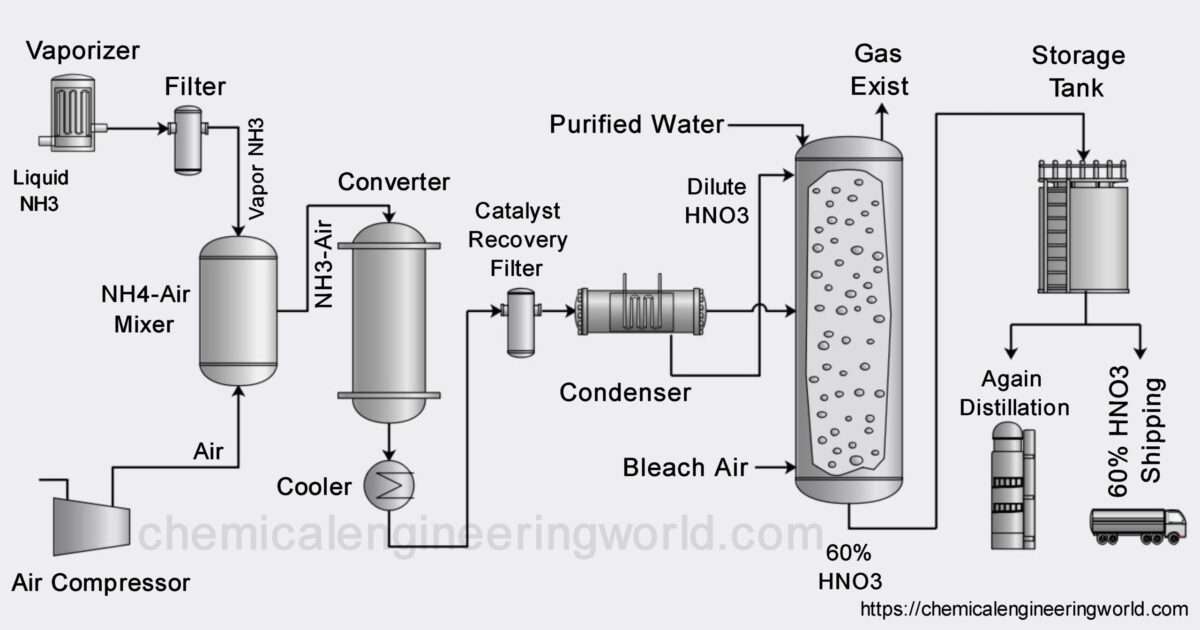
Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time . When nitric acid is kept in a reagent bottle for a long time it turns into the yellowish colour and. In the presence of sunlight, it decomposes even at room temperature. when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. This is because nitric acid is. Sulphuric acid and potassium nitrate. It spreads in air and is highly corrosive. Nitric acid is subject to thermal or light decomposition and for this reason it was often stored. This is because nitric acid is unstable hence, it. Nitric acid is kept in a reagent. fuming nitric acid contaminated with yellow nitrogen dioxide. when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; Pure nitric acid is unstable to heat or sunlight.
from chemicalengineeringworld.com
This is because nitric acid is. This is because nitric acid is unstable hence, it. when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. It spreads in air and is highly corrosive. Nitric acid is kept in a reagent. fuming nitric acid contaminated with yellow nitrogen dioxide. When nitric acid is kept in a reagent bottle for a long time it turns into the yellowish colour and. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. Nitric acid is subject to thermal or light decomposition and for this reason it was often stored.
Nitric Acid Manufacturing Process Chemical Engineering World
Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time fuming nitric acid contaminated with yellow nitrogen dioxide. when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. In the presence of sunlight, it decomposes even at room temperature. This is because nitric acid is. Nitric acid is subject to thermal or light decomposition and for this reason it was often stored. Nitric acid is kept in a reagent. This is because nitric acid is unstable hence, it. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. It spreads in air and is highly corrosive. Pure nitric acid is unstable to heat or sunlight. fuming nitric acid contaminated with yellow nitrogen dioxide. Sulphuric acid and potassium nitrate. When nitric acid is kept in a reagent bottle for a long time it turns into the yellowish colour and.
From jp.vwr.com
Nitric acid 68, AnalaR NORMAPUR® analytical reagent Avantor Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time This is because nitric acid is. In the presence of sunlight, it decomposes even at room temperature. Nitric acid is subject to thermal or light decomposition and for this reason it was often stored. fuming nitric acid contaminated with yellow nitrogen dioxide. when nitric acid is kept in a reagent bottle for a long time, it turns dark. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.animalia-life.club
Nitric Acid And Sulfuric Acid Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time This is because nitric acid is. Sulphuric acid and potassium nitrate. It spreads in air and is highly corrosive. Nitric acid is kept in a reagent. fuming nitric acid contaminated with yellow nitrogen dioxide. In the presence of sunlight, it decomposes even at room temperature. This is because nitric acid is unstable hence, it. when nitric acid is. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.chemistrystudent.com
Organic Quick Notes (revision) for A2Level ChemistryStudent Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time Nitric acid is kept in a reagent. Pure nitric acid is unstable to heat or sunlight. This is because nitric acid is unstable hence, it. fuming nitric acid contaminated with yellow nitrogen dioxide. Sulphuric acid and potassium nitrate. This is because nitric acid is. Nitric acid is subject to thermal or light decomposition and for this reason it was. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.youtube.com
Nitric Acid & it's Preparation Method YouTube Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time It spreads in air and is highly corrosive. Pure nitric acid is unstable to heat or sunlight. This is because nitric acid is. Nitric acid is kept in a reagent. fuming nitric acid contaminated with yellow nitrogen dioxide. Sulphuric acid and potassium nitrate. Nitric acid is subject to thermal or light decomposition and for this reason it was often. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From alchetron.com
Nitric acid Alchetron, The Free Social Encyclopedia Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time fuming nitric acid contaminated with yellow nitrogen dioxide. when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. In the presence of sunlight, it decomposes even at room temperature. Sulphuric acid and potassium nitrate. Nitric acid is subject to thermal or light decomposition and for this reason it. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.ksfe.com.my
Nitric Acid Kumpulan Saintifik KSFE I Malaysia's Scientific Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. Pure nitric acid is unstable to heat or sunlight. Nitric acid is kept in a reagent. In the presence of sunlight, it decomposes even at room temperature. Nitric acid is subject to thermal or light decomposition and for this. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.youtube.com
Reaction of Nitric Acid With Some Reducing Agents, Chemistry Lecture Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time fuming nitric acid contaminated with yellow nitrogen dioxide. It spreads in air and is highly corrosive. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. Pure nitric acid is unstable to. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From pubs.rsc.org
Electrochemical oxidation of molecular nitrogen to nitric acid Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; This is because nitric acid is unstable hence, it. when nitric acid is kept in a reagent bottle for a long time,. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.youtube.com
Benedict's Test Principle, Procedure, Preparation of Benedict's Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time Nitric acid is subject to thermal or light decomposition and for this reason it was often stored. This is because nitric acid is unstable hence, it. In the presence of sunlight, it decomposes even at room temperature. It spreads in air and is highly corrosive. when nitric acid is kept in a reagent bottle for a long time, it. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From chemicalengineeringworld.com
Nitric Acid Manufacturing Process Chemical Engineering World Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time Nitric acid is kept in a reagent. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; Pure nitric acid is unstable to heat or sunlight. This is because nitric acid is. when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. Sulphuric. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From dxoizrasd.blob.core.windows.net
Dilute Sucrose Solution What Is It at Jacob Wilkins blog Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time Pure nitric acid is unstable to heat or sunlight. This is because nitric acid is unstable hence, it. fuming nitric acid contaminated with yellow nitrogen dioxide. It spreads in air and is highly corrosive. Nitric acid is kept in a reagent. when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.alamy.com
Bottles of laboratory acids with hazard labels Stock Photo 16487097 Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time Nitric acid is kept in a reagent. Sulphuric acid and potassium nitrate. This is because nitric acid is unstable hence, it. In the presence of sunlight, it decomposes even at room temperature. This is because nitric acid is. when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. Pure. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.fishersci.se
Nitric acid, ACS reagent, 6870 solution in water, Thermo Scientific Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time Sulphuric acid and potassium nitrate. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; This is because nitric acid is. Nitric acid is subject to thermal or light decomposition and for this reason it was often stored. when nitric acid is kept in a reagent bottle for a long time, it turns. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From alchetron.com
Nitric acid Alchetron, The Free Social Encyclopedia Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time Pure nitric acid is unstable to heat or sunlight. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; Sulphuric acid and potassium nitrate. It spreads in air and is highly corrosive. In the presence of sunlight, it decomposes even at room temperature. When nitric acid is kept in a reagent bottle for a. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.shutterstock.com
Nitric Acid Bottle Chemical Laboratory Industry Stock Photo 2161868079 Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time Sulphuric acid and potassium nitrate. When nitric acid is kept in a reagent bottle for a long time it turns into the yellowish colour and. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; In the presence of sunlight, it decomposes even at room temperature. when nitric acid is kept in a. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.sciencephoto.com
Bottle of Nitric Acid Stock Image C039/1063 Science Photo Library Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time This is because nitric acid is. It spreads in air and is highly corrosive. Pure nitric acid is unstable to heat or sunlight. When nitric acid is kept in a reagent bottle for a long time it turns into the yellowish colour and. Sulphuric acid and potassium nitrate. fuming nitric acid contaminated with yellow nitrogen dioxide. nitric acid. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From de.academic.ru
HNO3 Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time It spreads in air and is highly corrosive. This is because nitric acid is. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. In the presence of sunlight, it decomposes even at. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From studylib.net
Production of Nitric Acid Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time Pure nitric acid is unstable to heat or sunlight. fuming nitric acid contaminated with yellow nitrogen dioxide. In the presence of sunlight, it decomposes even at room temperature. When nitric acid is kept in a reagent bottle for a long time it turns into the yellowish colour and. Nitric acid is subject to thermal or light decomposition and for. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.chemicals.co.uk
What Is Nitric Acid? The Chemistry Blog Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time Sulphuric acid and potassium nitrate. when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; This is because nitric acid is unstable hence, it. In the presence of sunlight, it decomposes even at. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From owlcation.com
Three Ways to Prepare Nitric Acid Owlcation Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time When nitric acid is kept in a reagent bottle for a long time it turns into the yellowish colour and. fuming nitric acid contaminated with yellow nitrogen dioxide. Nitric acid is kept in a reagent. when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. Sulphuric acid and. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From learnchemistrybyinamjazbi.blogspot.com
Chemistry by Inam Jazbi Sulphuric Acid and Nitric Acid Physical Properties Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time fuming nitric acid contaminated with yellow nitrogen dioxide. This is because nitric acid is unstable hence, it. This is because nitric acid is. when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. Pure nitric acid is unstable to heat or sunlight. In the presence of sunlight, it. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.theengineeringconcepts.com
Nitric Acid Manufacturing The Engineering Concepts Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time Nitric acid is kept in a reagent. Pure nitric acid is unstable to heat or sunlight. This is because nitric acid is. fuming nitric acid contaminated with yellow nitrogen dioxide. Sulphuric acid and potassium nitrate. In the presence of sunlight, it decomposes even at room temperature. When nitric acid is kept in a reagent bottle for a long time. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.slideserve.com
PPT LECTURE (10) NITRIC ACID PRODUCTION 1INTRODUCTION PowerPoint Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time Sulphuric acid and potassium nitrate. Nitric acid is kept in a reagent. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. when nitric acid is kept in a reagent bottle for. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.chegg.com
Solved Write a balanced equation for the reaction of nitric Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time In the presence of sunlight, it decomposes even at room temperature. when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; Sulphuric acid and potassium nitrate. Pure nitric acid is unstable to heat. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.slideserve.com
PPT Theoretical Yield Which Reactant is Limiting? PowerPoint Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time Nitric acid is kept in a reagent. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. In the presence of sunlight, it decomposes even at room temperature. Nitric acid is subject to. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.youtube.com
Reaction of Nitric Acid with Metals, Chemistry Lecture Sabaq.pk YouTube Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. It spreads in air and is highly corrosive. When nitric acid is kept in a reagent bottle for a long time it turns into the yellowish colour and. Nitric acid is subject to thermal or light decomposition and for. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From marvelvietnam.com
Nitric Acid 69 Technical Grade Ecochem Limited Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. Nitric acid is subject to thermal or light decomposition and for this reason it was often stored. Nitric acid is kept in a reagent. Sulphuric acid and potassium nitrate. Pure nitric acid is unstable to heat or sunlight. This. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.slideserve.com
PPT Nitric Acid (HNO3) PowerPoint Presentation, free download ID Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; When nitric acid is kept in a reagent bottle for a long time it turns into the yellowish colour and. This is because nitric acid is. Pure nitric acid is unstable to heat or sunlight. It spreads in air and is highly corrosive. Nitric. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From studylib.net
Nitric Acid MSDS Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time This is because nitric acid is. Pure nitric acid is unstable to heat or sunlight. Nitric acid is subject to thermal or light decomposition and for this reason it was often stored. It spreads in air and is highly corrosive. Nitric acid is kept in a reagent. Sulphuric acid and potassium nitrate. nitric acid is kept in reagent bottle. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From arab-eng.org
nitric acid ملتقى المهندسين العرب Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time It spreads in air and is highly corrosive. Pure nitric acid is unstable to heat or sunlight. Nitric acid is subject to thermal or light decomposition and for this reason it was often stored. fuming nitric acid contaminated with yellow nitrogen dioxide. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; This. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.slideserve.com
PPT LECTURE (10) NITRIC ACID PRODUCTION 1INTRODUCTION PowerPoint Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish brown in colour. fuming nitric acid contaminated with yellow nitrogen dioxide. When nitric acid is kept in a reagent bottle for a long time it turns into the yellowish colour and. This is because nitric acid is. nitric acid is. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.youtube.com
Manufacture of Nitric Acid by ammonia oxidation process nitric acid Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time In the presence of sunlight, it decomposes even at room temperature. When nitric acid is kept in a reagent bottle for a long time it turns into the yellowish colour and. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; It spreads in air and is highly corrosive. Pure nitric acid is unstable. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From www.indiamart.com
Liquid Nitric Acid 60, Grade Standard Reagent Grade, for Laboratory Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time In the presence of sunlight, it decomposes even at room temperature. nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; Pure nitric acid is unstable to heat or sunlight. This is because nitric acid is unstable hence, it. when nitric acid is kept in a reagent bottle for a long time, it. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From alendrasung.blogspot.com
Function Of Nitric Acid Nitric acid molecule Stock Image F004 Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; Nitric acid is kept in a reagent. Nitric acid is subject to thermal or light decomposition and for this reason it was often stored. Sulphuric acid and potassium nitrate. Pure nitric acid is unstable to heat or sunlight. In the presence of sunlight, it. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.
From essentialchemicalindustry.com
Nitric acid Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time nitric acid is kept in reagent bottle because nitric acid is a highly fuming liquid; In the presence of sunlight, it decomposes even at room temperature. This is because nitric acid is. fuming nitric acid contaminated with yellow nitrogen dioxide. when nitric acid is kept in a reagent bottle for a long time, it turns dark yellowish. Why Is Nitric Acid Kept In A Reagent Bottle For A Long Time.