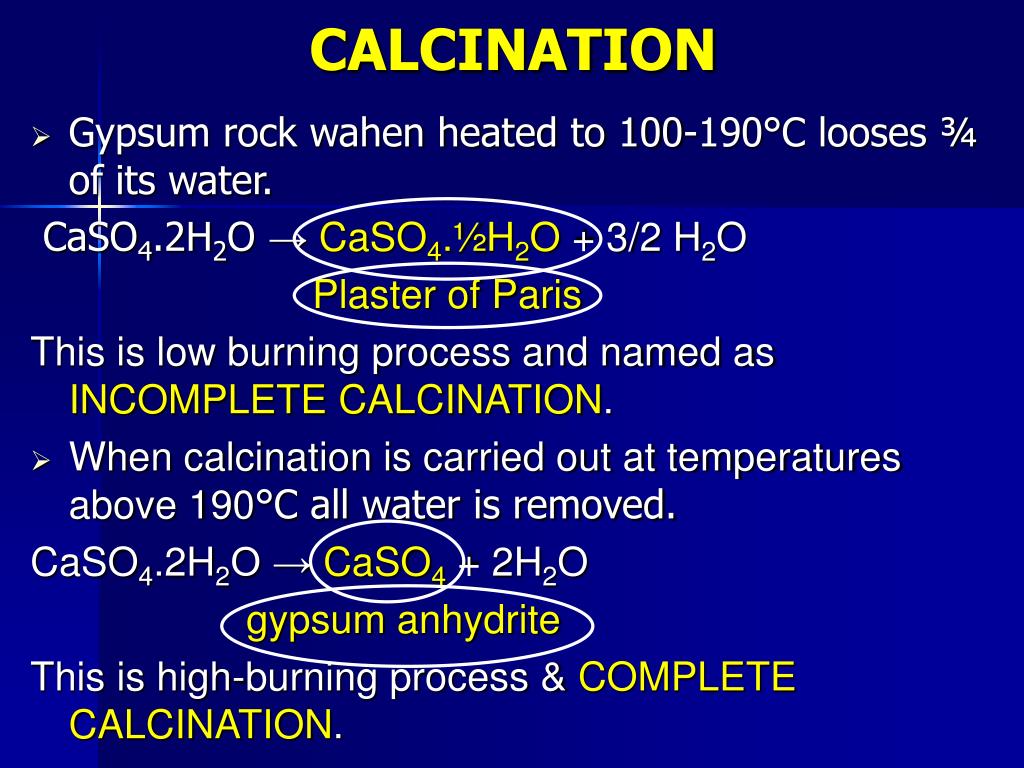
How Is Gypsum Calcined . gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. the calcination processes could be divided in two main categories [4]: calcination of gypsum. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. (i) direct heating processes in which gypsum. when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water of crystallization and is converted to. its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one.
from www.slideserve.com
when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water of crystallization and is converted to. gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. the calcination processes could be divided in two main categories [4]: its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. (i) direct heating processes in which gypsum. calcination of gypsum.
PPT GYPSUM PowerPoint Presentation, free download ID734258
How Is Gypsum Calcined Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water of crystallization and is converted to. its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. the calcination processes could be divided in two main categories [4]: the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. calcination of gypsum. gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. (i) direct heating processes in which gypsum.
From www.dreamstime.com
Gypsum Plaster, Also Called Plaster of Paris or Calcined Gypsum Stock How Is Gypsum Calcined the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. calcination of gypsum. its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. the calcination processes could be divided. How Is Gypsum Calcined.
From www.indiamart.com
White 100 Mesh Calcined Gypsum Powder, Packaging Type Pp And Jumbo Bag How Is Gypsum Calcined gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. calcination of gypsum. the calcination processes could be divided in two main categories [4]: (i) direct heating processes in which gypsum. the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. its chemical formula, caso4·2h2o, indicates that. How Is Gypsum Calcined.
From www.indiamart.com
Uthaya Calcined Gypsum, Packaging Type bag, Packaging Size 50 kg at How Is Gypsum Calcined the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water of crystallization and is converted to. Gypsum or calcium sulfate dihydrate (caso. How Is Gypsum Calcined.
From www.tradeindia.com
Calcined Gypsum Powder at Best Price in Udaipur, Rajasthan Ocean How Is Gypsum Calcined (i) direct heating processes in which gypsum. the calcination processes could be divided in two main categories [4]: gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. the preparation of natural and synthetic gypsum. How Is Gypsum Calcined.
From www.indiamart.com
Calcined Gypsum Powder, Packaging Size 25 Kg, Packaging Type Plastic How Is Gypsum Calcined its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. the calcination processes could be divided in two main categories [4]: calcination of gypsum. gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. the preparation of natural and synthetic gypsum prior to calcination,. How Is Gypsum Calcined.
From www.indiamart.com
White Calcined Gypsum Powder, For Construction at Rs 160/bag in Bhachau How Is Gypsum Calcined calcination of gypsum. (i) direct heating processes in which gypsum. the calcination processes could be divided in two main categories [4]: its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water. How Is Gypsum Calcined.
From www.indiamart.com
Calcined Gypsum Powder, Grade Standard Super Fine, Packaging Size How Is Gypsum Calcined calcination of gypsum. the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. (i) direct heating processes in which gypsum. the calcination processes could be divided in two main categories [4]: gypsum,. How Is Gypsum Calcined.
From www.zkg.de
System and method for manufacturing calcined gypsum with inline How Is Gypsum Calcined Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water of crystallization and is converted to. the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. . How Is Gypsum Calcined.
From www.grenzebach.com
Calcining processes for natural and synthetic gypsum Grenzebach How Is Gypsum Calcined its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. the calcination processes could be divided in two main categories [4]: (i) direct heating processes in which gypsum. when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water of crystallization and is. How Is Gypsum Calcined.
From www.researchgate.net
XRD patterns of (a) titanium gypsum; (b) calcined gypsum; (c) CaSO 4 How Is Gypsum Calcined Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. the calcination processes could be divided in two main categories [4]: the preparation of natural and synthetic gypsum prior to calcination, such as drying and. How Is Gypsum Calcined.
From www.indiamart.com
White Calcined Gypsum Powder, Packaging Type Bag, Packaging Size 50 How Is Gypsum Calcined (i) direct heating processes in which gypsum. its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. . How Is Gypsum Calcined.
From www.tradeindia.com
Calcined Gypsum Powder Common Cement at Best Price in Jodhpur How Is Gypsum Calcined gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. calcination of gypsum. its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. the preparation of. How Is Gypsum Calcined.
From www.jonoubgypsum.com
3 useful Gypsum Calcination kilns Jonoub Gypsum How Is Gypsum Calcined the calcination processes could be divided in two main categories [4]: calcination of gypsum. gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium. How Is Gypsum Calcined.
From www.mecrucn.com
Gypsum calcination process MECRU Heavy Industry Technology How Is Gypsum Calcined when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water of crystallization and is converted to. gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. calcination of gypsum. the calcination processes could be divided in two main categories [4]: its chemical formula, caso4·2h2o, indicates. How Is Gypsum Calcined.
From www.shutterstock.com
Gypsum Plaster Called Plaster Paris Calcined Stock Photo (Edit Now How Is Gypsum Calcined Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. gypsum, common sulfate mineral of. How Is Gypsum Calcined.
From nutrawiki.org
Calcined Gypsum NutraWiki How Is Gypsum Calcined when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water of crystallization and is converted to. gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. Gypsum or calcium sulfate dihydrate (caso. How Is Gypsum Calcined.
From www.mecrucn.com
Gypsum calcination process MECRU Heavy Industry Technology How Is Gypsum Calcined (i) direct heating processes in which gypsum. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. calcination of gypsum. the calcination processes could be divided in two main categories [4]: when. How Is Gypsum Calcined.
From www.youtube.com
gypsum calcined powder production line,plaster powder production line How Is Gypsum Calcined the calcination processes could be divided in two main categories [4]: the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. calcination of gypsum. (i) direct heating processes in which gypsum. gypsum,. How Is Gypsum Calcined.
From www.researchgate.net
Experimental gypsum calcination in two traditional kilns performed in How Is Gypsum Calcined when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water of crystallization and is converted to. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. the preparation. How Is Gypsum Calcined.
From www.indiamart.com
Calcined Gypsum Powder, 25 Kg, Packaging Type Bag at best price in Kolkata How Is Gypsum Calcined gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. (i) direct heating processes in which gypsum. its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. the calcination processes could be divided in two main categories [4]: when heated to a temperature of from. How Is Gypsum Calcined.
From www.researchgate.net
XRD patterns of (a) titanium gypsum; (b) calcined gypsum; (c) CaSO 4 How Is Gypsum Calcined calcination of gypsum. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. the calcination processes could be divided in two main categories [4]: its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. the preparation of natural and. How Is Gypsum Calcined.
From www.slideserve.com
PPT GYPSUM PowerPoint Presentation, free download ID734258 How Is Gypsum Calcined (i) direct heating processes in which gypsum. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water of crystallization and is converted to. gypsum, common sulfate mineral of great commercial importance, composed. How Is Gypsum Calcined.
From gosoor.com.eg
Calcined Gypsum Gosoor Marketplace How Is Gypsum Calcined the calcination processes could be divided in two main categories [4]: the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. (i) direct heating processes in which gypsum. when heated to a temperature. How Is Gypsum Calcined.
From www.indiamart.com
White Calcined Gypsum Plaster, 3mm at Rs 22/kg in Pimpri Chinchwad ID How Is Gypsum Calcined the calcination processes could be divided in two main categories [4]: the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. calcination of gypsum. gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium. How Is Gypsum Calcined.
From www.indiamart.com
White Calcined Gypsum Powder, For Construction at Rs 160/bag in Bhachau How Is Gypsum Calcined the calcination processes could be divided in two main categories [4]: (i) direct heating processes in which gypsum. when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water of crystallization and is converted to. the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. . How Is Gypsum Calcined.
From www.zkg.de
Grinding and calcining of gypsum with Pfeiffer grinding plants Cement How Is Gypsum Calcined when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water of crystallization and is converted to. its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined. How Is Gypsum Calcined.
From www.indiamart.com
White Calcined Gypsum Powder, Grade 200 Mesh, Packaging Size 25 Kg How Is Gypsum Calcined Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. the calcination processes could be divided in two main categories [4]: calcination of gypsum. its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. the preparation of natural and. How Is Gypsum Calcined.
From www.grenzebach.com
Calcining processes for natural and synthetic gypsum Grenzebach How Is Gypsum Calcined when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water of crystallization and is converted to. calcination of gypsum. the calcination processes could be divided in two main categories [4]: its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. (i). How Is Gypsum Calcined.
From www.youtube.com
GYPSUM CALCINATION PROCESS YouTube How Is Gypsum Calcined the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. (i) direct heating processes in which gypsum. when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water. How Is Gypsum Calcined.
From www.indiamart.com
Calcined Gypsum Powder, Packaging Type Plastic Bag at best price in Pune How Is Gypsum Calcined its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. the calcination processes could be divided in two main categories [4]: Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that. How Is Gypsum Calcined.
From www.zkg.de
Apparatus and method for calcination of gypsum Cement Lime Gypsum How Is Gypsum Calcined gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. (i) direct heating processes in which gypsum. the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. the calcination processes could be divided in two main categories [4]: its chemical formula, caso4·2h2o, indicates that each unit of gypsum. How Is Gypsum Calcined.
From www.zkg.de
Grinding and calcining of gypsum with Pfeiffer grinding plants Cement How Is Gypsum Calcined the calcination processes could be divided in two main categories [4]: Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. the preparation of natural and synthetic gypsum prior to. How Is Gypsum Calcined.
From www.indiamart.com
White 100 Mesh Calcined Gypsum Powder, Packaging Type Pp And Jumbo Bag How Is Gypsum Calcined when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water of crystallization and is converted to. the calcination processes could be divided in two main categories [4]: (i) direct heating processes in which gypsum. the preparation of natural and synthetic gypsum prior to calcination, such as drying and grinding. Gypsum. How Is Gypsum Calcined.
From yoshino-gypsum.com
Gypsum Board production flow YOSHINO GYPSUM How Is Gypsum Calcined its chemical formula, caso4·2h2o, indicates that each unit of gypsum is composed of one calcium (ca) atom, one. calcination of gypsum. gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. Gypsum or calcium sulfate dihydrate (caso 4.2h 2 o) is a mineral of calcium that is mined in various. the preparation of. How Is Gypsum Calcined.
From www.indiamart.com
Calcined Gypsum Powder at best price in Ghaziabad ID 24733498873 How Is Gypsum Calcined (i) direct heating processes in which gypsum. the calcination processes could be divided in two main categories [4]: when heated to a temperature of from 110°c to 120°c gypsum looses more than half its water of crystallization and is converted to. gypsum, common sulfate mineral of great commercial importance, composed of hydrated calcium sulfate. Gypsum or calcium. How Is Gypsum Calcined.